Abstract
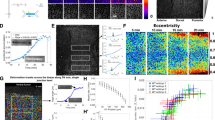
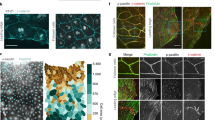
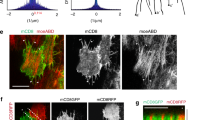
Embryonic wounds heal rapidly, in a process driven by coordinated cell movements. Polarization of actin and the molecular motor non-muscle myosin II in the cells adjacent to the wound results in the formation of a supracellular cable around the wound that drives repair. In Drosophila embryos, the distribution of actin around wounds is heterogeneous, with regions of high and low actin density, and actin heterogeneity is necessary for rapid repair. Here, we demonstrate that actin and myosin display stochastic patterns around embryonic wounds, and that contractile forces around wounds are heterogeneous. Mathematical modelling suggests that actomyosin heterogeneity favours wound closure if myosin is regulated by tension and strain, a hypothesis that we validate experimentally. We show that inhibition of stretch-activated ion channels disrupts myosin dynamics and tissue repair. Together, our results indicate that staggered contractile events, mechanical signals and force-regulated myosin dynamics coordinate cell behaviours to drive efficient wound closure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Martin, A. C., Gelbart, M., Fernandez-Gonzalez, R., Kaschube, M. & Wieschaus, E. F. Integration of contractile forces during tissue invagination. J. Cell Biol. 188, 735–749 (2010).
Fernandez-Gonzalez, R., Simoes Sde, M., Roper, J. C., Eaton, S. & Zallen, J. A. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736–743 (2009).
Lau, K. et al. Anisotropic stress orients remodelling of mammalian limb bud ectoderm. Nat. Cell Biol. 17, 569–579 (2015).
Rozbicki, E. et al. Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation. Nat. Cell Biol. 17, 397–408 (2015).
Williams-Masson, E. M., Malik, A. N. & Hardin, J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development 124, 2889–2901 (1997).
Kiehart, D. P., Galbraith, C. G., Edwards, K. A., Rickoll, W. L. & Montague, R. A. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 149, 471–490 (2000).
Martin, P. & Lewis, J. Actin cables and epidermal movement in embryonic wound healing. Nature 360, 179–183 (1992).
Wood, W. et al. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat. Cell Biol. 4, 907–912 (2002).
Davidson, L., Ezin, A. M. & Keller, R. Embryonic wound healing by apical contraction and ingression in Xenopus laevis. Cell Motil. Cytoskelet. 53, 163–176 (2002).
McCluskey, J., Hopkinson-Woolley, J., Luke, B. & Martin, P. A study of wound healing in the E11.5 mouse embryo by light and electron microscopy. Tissue Cell 25, 173–181 (1993).
Ducuing, A. & Vincent, S. The actin cable is dispensable in directing dorsal closure dynamics but neutralizes mechanical stress to prevent scarring in the Drosophila embryo. Nat. Cell Biol. 18, 1149–1160 (2016).
Brock, J., Midwinter, K., Lewis, J. & Martin, P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J. Cell Biol. 135, 1097–1107 (1996).
Abreu-Blanco, M. T., Verboon, J. M. & Parkhurst, S. M. Drosophila embryos close epithelial wounds using a combination of cellular protrusions and an actomyosin purse string. J. Cell Sci. 125, 5984–5997 (2012).
Fernandez-Gonzalez, R. & Zallen, J. A. Wounded cells drive rapid epidermal repair in the early Drosophila embryo. Mol. Biol. Cell 24, 3227–3237 (2013).
Zulueta-Coarasa, T., Tamada, M., Lee, E. J. & Fernandez-Gonzalez, R. Automated multidimensional image analysis reveals a role for Abl in embryonic wound repair. Development 141, 2901–2911 (2014).
Bement, W. M., Forscher, P. & Mooseker, M. S. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J. Cell Biol. 121, 565–578 (1993).
Brugués, A. et al. Forces driving epithelial wound healing. Nat. Phys. 10, 683–690 (2014).
Kiehart, D. P. & Feghali, R. Cytoplasmic myosin from Drosophila melanogaster. J. Cell Biol. 103, 1517–1525 (1986).
Martin, A. C., Kaschube, M. & Wieschaus, E. F. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499 (2009).
Hutson, M. S. et al. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science 300, 145–149 (2003).
Kumar, S. et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 90, 3762–3773 (2006).
Kovacs, M., Thirumurugan, K., Knight, P. J. & Sellers, J. R. Load-dependent mechanism of nonmuscle myosin 2. Proc. Natl Acad. Sci. USA 104, 9994–9999 (2007).
Kobb, A. B., Zulueta-Coarasa, T. & Fernandez-Gonzalez, R. Tension regulates myosin dynamics during Drosophila embryonic wound repair. J. Cell Sci. 130, 689–696 (2017).
Effler, J. C. et al. Mitosis-specific mechanosensing and contractile-protein redistribution control cell shape. Curr. Biol. 16, 1962–1967 (2006).
Yu, J. C. & Fernandez-Gonzalez, R. Local mechanical forces promote polarized junctional assembly and axis elongation in Drosophila. eLife 5, e10757 (2016).
Bowman, C. L., Gottlieb, P. A., Suchyna, T. M., Murphy, Y. K. & Sachs, F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon 49, 249–270 (2007).
Yang, X. C. & Sachs, F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science 243, 1068–1071 (1989).
Bardet, P. L. et al. A fluorescent reporter of caspase activity for live imaging. Proc. Natl Acad. Sci. USA 105, 13901–13905 (2008).
Hashimoto, H., Robin, F. B., Sherrard, K. M. & Munro, E. M. Sequential contraction and exchange of apical junctions drives zippering and neural tube closure in a simple chordate. Dev. Cell 32, 241–255 (2015).
Maddox, A. S., Lewellyn, L., Desai, A. & Oegema, K. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev. Cell 12, 827–835 (2007).
Niisato, N., Ohta, M., Eaton, D. C. & Marunaka, Y. Hypotonic stress upregulates beta- and gamma-ENaC expression through suppression of ERK by inducing MKP-1. Am. J. Physiol. Ren. Physiol. 303, F240–252 (2012).
Antunes, M., Pereira, T., Cordeiro, J. V., Almeida, L. & Jacinto, A. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. J. Cell Biol. 202, 365–379 (2013).
Xu, S. & Chisholm, A. D. A Gαq-Ca2+. signaling pathway promotes actin-mediated epidermal wound closure in C. elegans. Curr. Biol. 21, 1960–1967 (2011).
Hathaway, D. R. & Adelstein, R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc. Natl Acad. Sci. USA 76, 1653–1657 (1979).
Hartwig, J. H. et al. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature 356, 618–622 (1992).
Masiero, L., Lapidos, K. A., Ambudkar, I. & Kohn, E. C. Regulation of the RhoA pathway in human endothelial cell spreading on type IV collagen: role of calcium influx. J. Cell Sci. 112, 3205–3213 (1999).
Razzell, W., Evans, I. R., Martin, P. & Wood, W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr. Biol. 23, 424–429 (2013).
Hunter, M. V., Lee, D. M., Harris, T. J. & Fernandez-Gonzalez, R. Polarized E-cadherin endocytosis directs actomyosin remodeling during embryonic wound repair. J. Cell Biol. 210, 801–816 (2015).
Hunter, G. L., Crawford, J. M., Genkins, J. Z. & Kiehart, D. P. Ion channels contribute to the regulation of cell sheet forces during Drosophila dorsal closure. Development 141, 325–334 (2014).
Coste, B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 (2012).
Gudipaty, S. A. et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 543, 118–121 (2017).
Zhao, P. Y. et al. TRP channels localize to subdomains of the apical plasma membrane in human fetal retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 56, 1916–1923 (2015).
Simone, R. P. & DiNardo, S. Actomyosin contractility and Discs large contribute to junctional conversion in guiding cell alignment within the Drosophila embryonic epithelium. Development 137, 1385–1394 (2010).
Marcinkevicius, E. & Zallen, J. A. Regulation of cytoskeletal organization and junctional remodeling by the atypical cadherin Fat. Development 140, 433–443 (2013).
Tan, P. Y. & Zaidel-Bar, R. Transient membrane localization of SPV-1 drives cyclical actomyosin contractions in the C. elegans spermatheca. Curr. Biol. 25, 141–151 (2015).
Royou, A., Field, C., Sisson, J. C., Sullivan, W. & Karess, R. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol. Biol. Cell 15, 838–850 (2004).
Huang, J., Zhou, W. K., Dong, W., Watson, A. M. & Hong, Y. Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc. Natl Acad. Sci. USA 106, 8284–8289 (2009).
Oda, H. & Tsukita, S. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J. Cell Sci. 114, 493–501 (2001).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Foe, V. E. & Alberts, B. M. Studies of nuclear and cytoplasmic behavior during the 5 mitotic-cycles that precede gastrulation in Drosophila embryogenesis. J. Cell Sci. 61, 31–70 (1983).
Kiehart, D. P., Crawford, J. M. & Montague, R. A. Quantitative microinjection of Drosophila embryos: general strategy. CSH Protoc. 2007, pdbtop5 (2007).
Nishizawa, M. & Nishizawa, K. Molecular dynamics simulations of a stretch-activated channel inhibitor GsMTx4 with lipid membranes: two binding modes and effects of lipid structure. Biophys. J. 92, 4233–4243 (2007).
Hurst, A. C., Gottlieb, P. A. & Martinac, B. Concentration dependent effect of GsMTx4 on mechanosensitive channels of small conductance in E. coli spheroplasts. Eur. Biophys. J. 38, 415–425 (2009).
Fernandez-Gonzalez, R. & Zallen, J. A. Oscillatory behaviors and hierarchical assembly of contractile structures in intercalating cells. Phys. Biol. 8, 045005 (2011).
Leung, C. Y. & Fernandez-Gonzalez, R. Quantitative image analysis of cell behavior and molecular dynamics during tissue morphogenesis. Methods Mol. Biol. 1189, 99–113 (2015).
Fletcher, A. G., Osborne, J. M., Maini, P. K. & Gavaghan, D. J. Implementing vertex dynamics models of cell populations in biology within a consistent computational framework. Prog. Biophys. Mol. Biol. 113, 299–326 (2013).
Yu, J. C. & Fernandez-Gonzalez, R. Quantitative modelling of epithelial morphogenesis: integrating cell mechanics and molecular dynamics. Semin. Cell Dev. Biol. 67, 153–160 (2016).
Razzell, W., Wood, W. & Martin, P. Recapitulation of morphogenetic cell shape changes enables wound re-epithelialisation. Development 141, 1814–1820 (2014).
Lan, H., Wang, Q., Fernandez-Gonzalez, R. & Feng, J. J. A biomechanical model for cell polarization and intercalation during Drosophila germband extension. Phys. Biol. 12, 056011 (2015).
Merkel, R. et al. A micromechanic study of cell polarity and plasma membrane cell body coupling in Dictyostelium. Biophys. J. 79, 707–719 (2000).
Ren, Y. X. et al. Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr. Biol. 19, 1421–1428 (2009).
Koride, S. et al. Mechanochemical regulation of oscillatory follicle cell dynamics in the developing Drosophila egg chamber. Mol. Biol. Cell 25, 3709–3716 (2014).
Bambardekar, K., Clement, R., Blanc, O., Chardes, C. & Lenne, P. F. Direct laser manipulation reveals the mechanics of cell contacts in vivo. Proc. Natl Acad. Sci. USA 112, 1416–1421 (2015).
Glantz, S. A. Primer of Biostatistics (McGraw-Hill, New York, 2002).
Acknowledgements
We are grateful to T. Harris for sharing the myosin II antibody, to M. Hunter and J. Yu for technical assistance, and to C. Tong for help with data annotation. We thank A. McGuigan, C. Simmons and R. Winklbauer for useful discussions; and J. Feng, S. Hopyan, M. Hunter, A. Kobb, C. McFaul and J. Yu for comments on the manuscript. Flybase provided important information for this study. T.Z.-C. was supported by an Ontario Trillium Scholarship and a Doctoral Completion Award from the University of Toronto, and is a World Fellow of the Delta Kappa Gamma International Society. This work was supported by grants to R.F.-G. from the Natural Sciences and Engineering Research Council of Canada (418438-13), the Canada Foundation for Innovation (30279), the Ontario Ministry of Economic Development and Innovation (ER14-10-170), the Ted Rogers Centre for Heart Research TBEP Seed Program, and the Canada First Research Excellence Fund-University of Toronto Medicine by Design. R.F.-G. is the Tier II Canada Research Chair in Quantitative Cell Biology and Morphogenesis.
Author information
Authors and Affiliations
Contributions
T.Z.-C. and R.F.-G. developed the initial ideas for this study, analysed the data and prepared the manuscript. T.Z.-C. performed all experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Materials
Supplementary Figures 1–15, Supplementary Tables 1 and 2, Supplementary Video Legends 1–10, Supplementary References 1–3
Supplementary Video 1
Actin and myosin display similar patterns at the wound margin. Epidermal wound in an embryo co-expressing GFP:MoesinABD and myosin:mCherry. A stack was acquired every 30 s for 1 h and 39 min. Time after wounding is shown. Anterior left, dorsal up.
Supplementary Video 2
Mechanical forces at the wound margin are heterogeneous. Laser ablation experiments in embryos expressing E-cadherin:GFP. Cuts were performed in segments of the actomyosin cable with high (left) or low (right) myosin levels (see Fig. 2a,b). Yellow Xs indicate the sites of ablation. A stack was acquired every 3 s for 66 s. Time after ablation is shown. Anterior left, dorsal up.
Supplementary Video 3
Contraction is faster when the actomyosin distribution is heterogeneous. Heterogeneous (left) and homogeneous (right) patches of the wound margin in embryos expressing E-cadherin:mTomato (green) and myosin:GFP (magenta). Yellow arrowheads indicate the tracked segment. A stack was acquired every 10 s for 1 min.
Supplementary Video 4
Heterogeneous myosin distribution and mechanically regulated dynamics favour rapid wound closure in silico. Wound healing simulations with a heterogeneous myosin distribution and no myosin turnover (left), with tension-based myosin stabilization (centre-left), with strain-based myosin recruitment (centre), or with strain and tension-dependent myosin dynamics (centre-right); or with a homogeneous myosin distribution and strain and tension dependent myosin dynamics (right). Colour indicates interfacial tension in nN based on myosin levels (see Fig. 4 for scale). The simulation time step was 2 s for 20 min. Time is with respect to the onset of wound closure. Anterior left, dorsal up.
Supplementary Video 5
Non-uniform wound edge deformation is associated with embryonic wound repair. Strain maps of the wound edge during embryonic repair. Colour indicates the degree and the type of strain (tensile, cold colours; compressive, warm colours). A stack was acquired every 10 s for 20.3 min. Time after wounding is shown. Anterior left, dorsal up.
Supplementary Video 6
Myosin accumulates at a wound edge segment after being stretched. Relative timing between the deformation of wound edge segment and recruitment of myosin in an embryo expressing E-cadherin:mTomato (green) and myosin:GFP (magenta). Yellow arrowheads indicate the segment of interest. A stack was acquired every 10 s for 20.3 min. Time after wounding is shown. Anterior left, dorsal up.
Supplementary Video 7
Segments mechanically isolated from their neighbours fail to recruit myosin. Wound edge segments in embryos expressing E-cadherin:mTomato (green) and myosin:GFP (magenta) far from the site of wound edge ablation (left) or in a mechanically isolated segment (right). Yellow arrowheads indicate the segments in which fluorescence was monitored. Cyan Xs indicate the segments targeted for ablation. A stack was acquired every 30 s for 20.5 min. Time after segment isolation is shown. Anterior left, dorsal up.
Supplementary Video 8
Tensile strain is sufficient to recruit myosin. Cell interfaces in embryos expressing E-cadherin:mTomato (green) and myosin:GFP (magenta) in sham (left) or UV-irradiated 32 embryos (right). Yellow arrowheads indicate the monitored interfaces. Cyan Xs indicate the cells irradiated to induce strain. A stack was acquired every 10 s for 20.3 min. Time after wounding is shown. Anterior left, dorsal up.
Supplementary Video 9
GsMTx4 treatment impairs wound closure. Wounds in embryos expressing myosin:GFP and injected with buffer (left) or 2.5 mM GsMTx4 (right). A stack was acquired every 30 s for 39 min. Time after injection is shown. Anterior left, dorsal up.
Supplementary Video 10
GdCl3 injection prevents wound repair. Wounds in embryos expressing myosin:GFP and injected with water (left) or 20 mM GdCl3 (right). A stack was acquired every 30 s for 39.5 min. Time after injection is shown. Anterior left, dorsal up.
Rights and permissions
About this article
Cite this article
Zulueta-Coarasa, T., Fernandez-Gonzalez, R. Dynamic force patterns promote collective cell movements during embryonic wound repair. Nature Phys 14, 750–758 (2018). https://doi.org/10.1038/s41567-018-0111-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-018-0111-2
This article is cited by
-
Patterning of the cell cortex by Rho GTPases
Nature Reviews Molecular Cell Biology (2024)
-
Biomechanical, biophysical and biochemical modulators of cytoskeletal remodelling and emergent stem cell lineage commitment
Communications Biology (2023)
-
Motility-induced fracture reveals a ductile-to-brittle crossover in a simple animal’s epithelia
Nature Physics (2021)
-
Collective migrations in an epithelial–cancerous cell monolayer
Acta Mechanica Sinica (2021)
-
Stability bounds of a delay visco-elastic rheological model with substrate friction
Journal of Mathematical Biology (2021)