Abstract
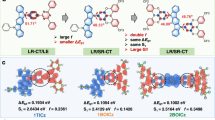
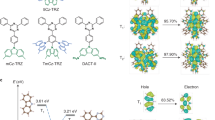
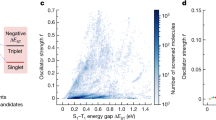
Reverse intersystem crossing (RISC), originally considered forbidden in purely organic materials, has recently become possible by minimizing the energy gap between the lowest excited singlet state (S1) and lowest triplet state (T1) in thermally activated delayed fluorescence systems. However, direct spin-inversion from T1 to S1 is still inefficient when both states are of the same charge transfer (CT) nature (that is, 3CT and 1CT, respectively). Intervention of locally excited triplet states (3LE) between 3CT and 1CT is expected to trigger fast spin-flipping. Here, we report the systematic design of ideal thermally activated delayed fluorescence molecules with near-degenerate 1CT, 3CT and 3LE states by controlling the distance between the donor and acceptor segments in a molecule with tilted intersegment angles. This system realizes very fast RISC with a rate constant (kRISC) of 1.2 × 107 s−1, resulting in organic light-emitting diodes with excellent performance, particularly at high brightness.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Baldo, M. A., Lamansky, S., Burrows, P. E., Thompson, M. E. & Forrest, S. R. Very high-efficiency green organic light-emitting devices based on electrophosphorescence. Appl. Phys. Lett. 75, 4–6 (1999).
Sasabe, H. & Kido, J. Recent progress in phosphorescent organic light-emitting devices. Eur. J. Org. Chem. 2013, 7653–7663 (2013).
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).
Onoue, Y., Hiraki, K. & Nishikawa, Y. Interactions of solid supports and fluorescent substances in thermally activated delayed fluorescence. Anal. Sci. 3, 509–513 (1987).
Yang, Z. et al. Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 46, 915–1016 (2017).
Wong, M. Y. & Zysman-Colman, E. Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv. Mater. 29, 1605444 (2017).
Huang, T., Jiang, W. & Duan, L. Recent progress in solution processable TADF materials for organic light-emitting diodes. J. Mater. Chem. C 6, 5577–5596 (2018).
Endo, A. et al. Thermally activated delayed fluorescence from Sn4+-porphyrin complexes and their application to organic light emitting diodes—a novel mechanism for electroluminescence. Adv. Mater. 21, 4802–4806 (2009).
Zhu, Z.-Q., Fleetham, T., Turner, E. & Li, J. Harvesting all electrogenerated excitons through metal assisted delayed fluorescent materials. Adv. Mater. 27, 2533–2537 (2015).
Yersin, H., Czerwieniec, R., Shafikov, M. Z. & Suleymanova, A. F. TADF material design: photophysical background and case studies focusing on CuI and AgI complexes. ChemPhysChem 18, 3508–3535 (2017).
Di, D. et al. High-performance light-emitting diodes based on carbene-metal-amides. Science 356, 159–163 (2017).
To, W.-P. et al. Highly luminescent pincer gold(III) aryl emitters: thermally activated delayed fluorescence and solution-processed OLEDs. Angew. Chem. Int. Ed. 56, 14036–14041 (2017).
Hamze, R. et al. Eliminating nonradiative decay in Cu(I) emitters: >99% quantum efficiency and microsecond lifetime. Science 363, 601–606 (2019).
Kaji, H. et al. Purely organic electroluminescent material realizing 100% conversion from electricity to light. Nat. Commun. 6, 8476 (2015).
Lin, T.-A. et al. Sky-blue organic light emitting diode with 37% external quantum efficiency using thermally activated delayed fluorescence from spiroacridine-triazine hybrid. Adv. Mater. 28, 6976–6983 (2016).
El-Sayed, M. A. Spin–orbit coupling and the radiationless processes in nitrogen heterocyclics. J. Chem. Phys. 38, 2834–2838 (1963).
Marian, C. M. Mechanism of the triplet-to-singlet upconversion in the assistant dopant ACRXTN. J. Phys. Chem. C 120, 3715–3721 (2016).
Dias, F. B. et al. The role of local triplet excited states and D-A relative orientation in thermally activated delayed fluorescence: photophysics and devices. Adv. Sci. 3, 1600080 (2016).
Gibson, J., Monkman, A. P. & Penfold, T. J. The importance of vibronic coupling for efficient reverse intersystem crossing in thermally activated delayed fluorescence molecules. ChemPhysChem 17, 2956–2961 (2016).
Etherington, M. K., Gibson, J., Higginbotham, H. F., Penfold, T. J. & Monkman, A. P. Revealing the spin–vibronic coupling mechanism of thermally activated delayed fluorescence. Nat. Commun. 7, 13680 (2016).
Lyskov, I. & Marian, C. M. Climbing up the ladder: intermediate triplet states promote the reverse intersystem crossing in the efficient TADF emitter ACRSA. J. Phys. Chem. C 121, 21145–21153 (2017).
Hosokai, T. et al. Evidence and mechanism of efficient thermally activated delayed fluorescence promoted by delocalized excited states. Sci. Adv. 3, e1603282 (2017).
Samanta, P. K., Kim, D., Coropceanu, V. & Brédas, J.-L. Up-conversion intersystem crossing rates in organic emitters for thermally activated delayed fluorescence: impact of the nature of singlet vs triplet excited states. J. Am. Chem. Soc. 139, 4042–4051 (2017).
Noda, H., Nakanotani, H. & Adachi, C. Excited state engineering for efficient reverse intersystem crossing. Sci. Adv. 4, eaao6910 (2018).
Kawasumi, K. et al. Thermally activated delayed fluorescence materials based on homoconjugation effect of donor-acceptor triptycenes. J. Am. Chem. Soc. 137, 11908–11911 (2015).
Shao, S. et al. Blue thermally activated delayed fluorescence polymers with nonconjugated backbone and through-space charge transfer effect. J. Am. Chem. Soc. 139, 17739–17742 (2017).
Tsujimoto, H. et al. Thermally activated delayed fluorescence and aggregation induced emission with through-space charge transfer. J. Am. Chem. Soc. 139, 4894–4900 (2017).
Frisch, M. J. et al. Gaussian 16 revision B.01 (Gaussian Inc., 2016).
Sun, H., Zhong, C. & Brédas, J.-L. Reliable prediction with tuned range-separated functionals of the singlet-triplet gap in organic emitters for thermally activated delayed fluorescence. J. Chem. Theory Comput. 11, 3851–3858 (2015).
te Velde, G. et al. Chemistry with ADF. J. Comput. Chem. 22, 931–967 (2001).
Akinaga, Y. & Ten-no, S. Range-separation by the Yukawa potential in long-range corrected density functional theory with Gaussian-type basis functions. Chem. Phys. Lett. 462, 348–351 (2008).
Tsai, W.-L. et al. A versatile thermally activated delayed fluorescence emitter for both highly efficient doped and non-doped organic light emitting devices. Chem. Commun. 51, 13662–13665 (2015).
Wada, Y., Kubo, S. & Kaji, H. Adamantyl substitution strategy for realizing solution-processable thermally stable deep-blue thermally activated delayed fluorescence materials. Adv. Mater. 30, 1705641 (2018).
Olivier, Y. et al. Nature of the singlet and triplet excitations mediating thermally activated delayed fluorescence. Phys. Rev. Mater. 1, 075602 (2017).
Seo, J.-A. et al. Improved efficiency and stable lifetime in blue phosphorescent organic light-emitting diodes using a stable exciton blocking layer. Dyes Pigm. 123, 254–256 (2015).
Nakanotani, H. et al. High-efficiency organic light-emitting diodes with fluorescent emitters. Nat. Commun. 5, 4016 (2014).
Lee, I. H., Song, W., Lee, J. Y. & Hwang, S.-H. High efficiency blue fluorescent organic light-emitting diodes using a conventional blue fluorescent emitter. J. Mater. Chem. C 3, 8834–8838 (2015).
Song, W., Lee, I. & Lee, J. Y. Host engineering for high quantum efficiency blue and white fluorescent organic light-emitting diodes. Adv. Mater. 27, 4358–4363 (2015).
Han, S. H. & Lee, J. Y. Spatial separation of sensitizer and fluorescent emitter for high quantum efficiency in hyperfluorescent organic light-emitting diodes. J. Mater. Chem. C 6, 1504–1508 (2018).
Jeon, S. K., Park, H.-J. & Lee, J. Y. Highly efficient soluble blue delayed fluorescent and hyperfluorescent organic light-emitting diodes by host engineering. ACS Appl. Mater. Interfaces 10, 5700–5705 (2018).
Goushi, K., Yoshida, K., Sato, K. & Adachi, C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photon. 6, 253–258 (2012).
Acknowledgements
This work was supported by JSPS KAKENHI grant numbers 17H01231 (to H.K.) and 17J09631 (to Y.Wada). Computation time was provided by the Super Computer System, Institute for Chemical Research, Kyoto University. NMR measurements were supported by the international Joint Usage/Research Centre (iJURC) at the Institute for Chemical Research, Kyoto University, Japan. We thank A. Jackson for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
Y.Wada led the theoretical calculations, did the data curation, formal analysis, investigation, validation and visualization, and wrote the original draft of the manuscript, as well as supporting the conceptualization. H.N. led the synthesis, and supported the data curation, investigation and writing of the original draft, as well as the conceptualization. S.M. equally contributed to the data curation and validated the TAF system. Y.Wakisaka supported the conceptualization and formal analysis. H.K. proposed the original concept of the tFFO design, did the formal analysis, theoretical analysis of rate constants, visualization, the writing of the original draft, and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary text (material synthesis and characterization, theoretical calculations, thermal and photophysical properties), scheme 1, 1H and 13C NMR data, Figs. 1–7 and Tables 1–7.
Rights and permissions
About this article
Cite this article
Wada, Y., Nakagawa, H., Matsumoto, S. et al. Organic light emitters exhibiting very fast reverse intersystem crossing. Nat. Photonics 14, 643–649 (2020). https://doi.org/10.1038/s41566-020-0667-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-020-0667-0
This article is cited by
-
Onion-like multicolor thermally activated delayed fluorescent carbon quantum dots for efficient electroluminescent light-emitting diodes
Nature Communications (2024)
-
Entropy-driven charge-transfer complexation yields thermally activated delayed fluorescence and highly efficient OLEDs
Nature Chemistry (2024)
-
A figure of merit for efficiency roll-off in TADF-based organic LEDs
Nature (2024)
-
Enhancing the efficiency and stability of blue thermally activated delayed fluorescence emitters by perdeuteration
Nature Photonics (2024)
-
Key requirements for ultraefficient sensitization in hyperfluorescence organic light-emitting diodes
Nature Photonics (2024)