Abstract
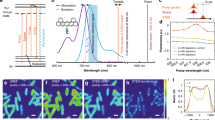
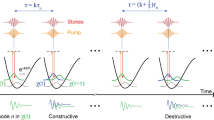
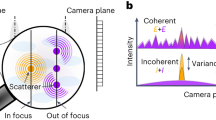
Coherent Raman scattering (for example, coherent anti-Stokes Raman scattering (CARS) and stimulated Raman scattering) microscopy has emerged as a powerful tool for label-free biomolecular imaging in biological and biomedical systems, but its spatial resolution is diffraction limited. Here, we report a higher-order coherent anti-Stokes Raman scattering (HO-CARS) microscopy to break the diffraction limit for label-free, super-resolution vibrational imaging. The resolution enhancement of HO-CARS microscopy has been analysed and demonstrated in biological samples (for example, live HeLa and buccal cells). The HO-CARS technique provides an inherent high resonant to non-resonant background ratio compared with conventional CARS microscopy. We affirm that under a tight focusing, the HO-CARS signal originating from the higher-order nonlinear process (χ(5), χ(7)) dominates over the cascaded lower-order nonlinear process (χ(3)), yielding much richer spectroscopic information. This study illustrates that HO-CARS microscopy can be an appealing tool for label-free, super-resolution imaging in biological and biomedical systems with high image contrast.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Zumbusch, A., Holtom, G. R. & Xie, X. S. Three-dimensional vibrational imaging by coherent anti-Stokes Raman scattering. Phys. Rev. Lett. 82, 4142–4145 (1999).
Evans, C. L. & Xie, X. S. Coherent anti-Stokes Raman scattering microscopy: chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 1, 883–909 (2008).
Freudiger, C. W. et al. Label-Free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008).
Cheng, J. X. & Xie, X. S. Vibrational spectroscopic imaging of living systems: an emerging platform for biology and medicine. Science 350, aaa8870 (2015).
Lu, F., Zheng, W., Sheppard, C. & Huang, Z. Interferometric polarization coherent anti-Stokes Raman scattering (IP-CARS) microscopy. Opt. Lett. 33, 602–604 (2008).
Lin, J., Lu, F., Zheng, W. & Huang, Z. Annular aperture-detected coherent anti-Stokes Raman scattering microscopy for high contrast vibrational imaging. Appl. Phys. Lett. 97, 083701 (2010).
Wang, Z., Zheng, W. & Huang, Z. Lock-in-detection-free line-scan stimulated Raman scattering microscopy for near video-rate Raman imaging. Opt. Lett. 41, 3960–3963 (2016).
Hell, S. W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–796 (2006).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Gustafsson, M. G. L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Fujita, K., Kobayashi, M., Kawano, S., Yamanaka, M. & Kawata, S. High-resolution confocal microscopy by saturated excitation of fluorescence. Phys. Rev. Lett. 99, 228105 (2007).
Prince, R. C. & Potma, E. O. Going visible: high-resolution coherent Raman imaging of cells and tissues. Light Sci. Appl. 8, 10 (2019).
Beeker, W. P. et al. A route to sub-diffraction-limited CARS microscopy. Opt. Express 17, 22632–22638 (2009).
Cleff, C. et al. Ground-state depletion for subdiffraction-limited spatial resolution in coherent anti-Stokes Raman scattering microscopy. Phys. Rev. A 86, 023825 (2012).
Gong, L. & Wang, H. Breaking the diffraction limit by saturation in stimulated-Raman-scattering microscopy: a theoretical study. Phys. Rev. A 90, 013818 (2014).
Gong, L. & Wang, H. Suppression of stimulated Raman scattering by an electromagnetically-induced-transparency-like scheme and its application for super-resolution microscopy. Phys. Rev. A 92, 023828 (2015).
Yonemaru, Y. et al. Super-spatial- and -spectral-resolution in vibrational imaging via saturated coherent anti-Stokes Raman scattering. Phys. Rev. Appl. 4, 014010 (2015).
Silva, W. R., Graefe, C. T. & Frontiera, R. R. Toward label-free super-resolution microscopy. ACS Photon. 3, 79–86 (2016).
Choi, D. S. et al. Selective suppression of CARS signal with three-beam competing stimulated Raman scattering processes. Phys. Chem. Chem. Phys. 20, 17156–17170 (2018).
Kim, D. et al. Selective suppression of stimulated Raman scattering with another competing stimulated Raman scattering. J. Phys. Chem. Lett. 8, 6118–6123 (2017).
Gong, L., Zheng, W., Ma, Y. & Huang, Z. Saturated stimulated Raman scattering microscopy for far-field super-resolution bioimaging. Phys. Rev. Appl. 11, 034041 (2019).
Gong, L. et al. Supercritical focusing coherent anti-Stokes Raman scattering microscopy for high-resolution vibrational imaging. Opt. Lett. 43, 5615–5618 (2018).
Kim, H., Bryant, G. W. & Stranick, S. J. Superresolution four-wave mixing microscopy. Opt. Express 20, 6042–6051 (2012).
Boyd, R. W. Nonlinear Optics 3rd edn (Academic Press, Elsevier, 2010).
Kawashima, Y. & Katagiri, G. Fundamentals, overtones, and combinations in the Raman spectrum of graphite. Phys. Rev. B 52, 10053–10059 (1995).
Compaan, A., Wiener-Avnear, E. & Chandra, S. Second-order coherent Raman scattering. Phys. Rev. A 17, 1083–1092 (1978).
Mukamel, S. Multidimensional femtosecond correlation spectroscopies of electronic and vibrational excitations. Annu. Rev. Phys. Chem. 51, 691–729 (2000).
Pelegati, V. B., Kyotoku, B. B. C., Padilha, L. A. & Cesar, C. L. Six-wave mixing coherent anti-Stokes Raman scattering microscopy. Biomed. Opt. Express 9, 2407–2417 (2018).
Blank, D. A., Kaufman, L. J. & Fleming, G. R. Fifth-order two-dimensional Raman spectra of CS2 are dominated by third-order cascades. J. Chem. Phys. 111, 3105–3114 (1999).
Kano, H. & Hamaguchi, H. Cascading third-order Raman process studied by six-wave mixing broadband multiplex coherent anti-Stokes Raman scattering spectroscopy. J. Chem. Phys. 118, 4556–4562 (2003).
Guo, Z., Molesky, B. P., Cheshire, T. P. & Moran, A. M. Two-dimensional resonance Raman signatures of vibronic coherence transfer in chemical reactions. Top. Curr. Chem. 375, 87 (2017).
Bae, K. et al. Epi-detected hyperspectral stimulated Raman scattering microscopy for label-free molecular subtyping of glioblastomas. Anal. Chem. 90, 10249–10255 (2018).
Bae, K., Zheng, W., Ma, Y. & Huang, Z. Real-time monitoring of pharmacokinetics of antibiotics in biofilms with Raman-tagged hyperspectral stimulated Raman scattering microscopy. Theranostics 9, 1348–1357 (2019).
Lin, K., Zheng, W., Lim, C. M. & Huang, Z. Real-time in vivo diagnosis of nasopharyngeal carcinoma using rapid fiber-optic Raman spectroscopy. Theranostics 7, 3517–3526 (2017).
Wang, H., Fu, Y. & Cheng, J. X. Experimental observation and theoretical analysis of Raman resonance-enhanced photodamage in coherent anti-Stokes Raman scattering microscopy. J. Opt. Soc. Am. B 24, 544–552 (2007).
Fu, Y., Wang, H., Shi, R. & Cheng, J.-X. Characterization of photodamage in coherent anti-Stokes Raman scattering microscopy. Opt. Express 14, 3942–3951 (2006).
König, K., Becker, T. W., Fischer, P., Riemann, I. & Halbhuber, K. J. Pulse-length dependence of cellular response to intense near-infrared laser pulses in multiphoton microscopes. Opt. Lett. 24, 113–115 (1999).
Bi, Y. et al. Near-resonance enhanced label-free stimulated Raman scattering microscopy with spatial resolution near 130 nm. Light Sci. Appl. 7, 81 (2018).
Lu, F. K. et al. Label-free DNA imaging in vivo with stimulated Raman scattering microscopy. Proc. Natl Acad. Sci. USA 112, 11624–11629 (2015).
Gough, K. M. & Henry, B. R. Gas-phase overtone spectral investigation of inequivalent aryl and alkyl carbon-hydrogen (C–H) bonds in toluene and the xylenes. J. Phys. Chem. 88, 1298–1302 (1984).
Amrein, A., Dübal, H. R. & Quack, M. Multiple anharmonic resonances in the vibrational overtone spectra of CHClF2. Mol. Phys. 56, 727–735 (1985).
Angioni, E. et al. UV spectral properties of lipids as a tool for their identification. Eur. J. Lipid. Sci. Tech. 104, 59–64 (2002).
Pace, C. N., Vajdos, F., Fee, L., Grimsley, G. & Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 4, 2411–2423 (1995).
Acknowledgements
This work was supported by the Academic Research Fund (AcRF)-Tier 1 and Tier 2 from Ministry of Education (MOE) (MOE2014-T2-1-010), and the National Medical Research Council (NMRC) (NMRC/TCR/016-NNI/2016), Singapore.
Author information
Authors and Affiliations
Contributions
L.G. and Z.H. conceived the concept and designed the experiments. L.G. performed the experiments. Y.M. performed chemical synthesis. L.G., W.Z. and Z.H. performed the data analysis and wrote the manuscript. Z.H. finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gong, L., Zheng, W., Ma, Y. et al. Higher-order coherent anti-Stokes Raman scattering microscopy realizes label-free super-resolution vibrational imaging. Nat. Photonics 14, 115–122 (2020). https://doi.org/10.1038/s41566-019-0535-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-019-0535-y
This article is cited by
-
Probing delivery of a lipid nanoparticle encapsulated self-amplifying mRNA vaccine using coherent Raman microscopy and multiphoton imaging
Scientific Reports (2024)
-
Confocal nonlinear optical imaging on hexagonal boron nitride nanosheets
PhotoniX (2023)
-
Super-resolution imaging of non-fluorescent molecules by photothermal relaxation localization microscopy
Nature Photonics (2023)
-
Label-free biomedical optical imaging
Nature Photonics (2023)
-
Far-field super-resolution chemical microscopy
Light: Science & Applications (2023)