Abstract
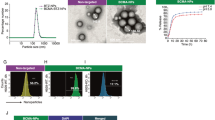
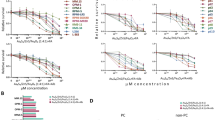
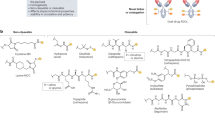
Cancer therapies often have narrow therapeutic indexes and involve potentially suboptimal combinations due to the dissimilar physical properties of drug molecules. Nanomedicine platforms could address these challenges, but it remains unclear whether synergistic free-drug ratios translate to nanocarriers and whether nanocarriers with multiple drugs outperform mixtures of single-drug nanocarriers at the same dose. Here we report a bottlebrush prodrug (BPD) platform designed to answer these questions in the context of multiple myeloma therapy. We show that proteasome inhibitor (bortezomib)-based BPD monotherapy slows tumour progression in vivo and that mixtures of bortezomib, pomalidomide and dexamethasone BPDs exhibit in vitro synergistic, additive or antagonistic patterns distinct from their corresponding free-drug counterparts. BPDs carrying a statistical mixture of three drugs in a synergistic ratio outperform the free-drug combination at the same ratio as well as a mixture of single-drug BPDs in the same ratio. Our results address unanswered questions in the field of nanomedicine, offering design principles for combination nanomedicines and strategies for improving current front-line monotherapies and combination therapies for multiple myeloma.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information and can also be obtained from the corresponding authors upon reasonable request.
Change history
13 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41565-023-01345-y
10 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41565-023-01499-9
References
Tibbitt, M. W., Dahlman, J. E. & Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 138, 704–717 (2016).
Shi, J., Kantoff, P. W., Wooster, R. & Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2017).
Shi, J., Xiao, Z., Kamaly, N. & Farokhzad, O. C. Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation. Acc. Chem. Res. 44, 1123–1134 (2011).
Kakkar, A., Traverso, G., Farokhzad, O. C., Weissleider, R. & Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 1, 0063 (2017).
Ma, L., Kohli, M. & Smith, A. Nanoparticles for combination drug therapy. ACS Nano 7, 9518–9525 (2013).
Mignani, S., Bryszewska, M., Klajnert-Maculewicz, B., Zablocka, M. & Majoral, J.-P. Advances in combination therapies based on nanoparticles for efficacious cancer treatment: an analytical report. Biomacromolecules 16, 1–27 (2015).
Zhang, R. X., Wong, H. L., Xue, H. Y., Eoh, J. Y. & Wu, X. Y. Nanomedicine of synergistic drug combinations for cancer therapy—strategies and perspectives. J. Control. Release 240, 489–503 (2016).
Hu, Q., Sun, W., Wang, C. & Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 98, 19–34 (2016).
Shim, G., Kim, M.-G., Kim, D., Park, J. Y. & Oh, Y.-K. Nanoformulation-based sequential combination cancer therapy. Adv. Drug Deliv. Rev. 115, 57–81 (2017).
Jia, J. et al. Mechanisms of drug combinations: interaction and network perspectives. Nat. Rev. Drug Discov. 8, 111–128 (2009).
Tardi, P. et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk. Res. 33, 129–139 (2009).
Batist, G. et al. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin. Cancer Res. 15, 692–700 (2009).
Lehar, J. et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 27, 659–666 (2009).
Kolishetti, N. et al. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl Acad. Sci. USA 107, 17939–17944 (2010).
Deng, Z. J. et al. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 7, 9571–9584 (2013).
Aryal, S., Hu, C.-M. J. & Zhang, L. Polymeric nanoparticles with precise ratiometric control over drug loading for combination therapy. Mol. Pharmaceutics 8, 1401–1407 (2011).
Lammers, T. et al. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials 30, 3466–3475 (2009).
Wang, H. et al. Precise engineering of prodrug cocktails into single polymeric nanoparticles for combination cancer therapy: extended and sequentially controllable drug release. ACS Appl. Mater. Interfaces 9, 10567–10576 (2017).
Zhang, L. et al. Enhancing solid tumor therapy with sequential delivery of dexamethasone and docetaxel engineered in a single carrier to overcome stromal resistance to drug delivery. J. Control. Release 294, 1–16 (2019).
Cai, L. et al. Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: a synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials 37, 456–468 (2015).
Howlader, N. et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute, Bethesda, MD, based on November 2015 SEER data submission, posted to the SEER website (2016); https://seer.cancer.gov/archive/csr/1975_2013/
Attal, M. et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N. Engl. J. Med. 376, 1311–1320 (2017).
Nooka, A. K. et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia 28, 690–693 (2014).
Richardson, P. G. et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 20, 781–794 (2019).
Chanan-Khan, A. A. et al. Pomalidomide: the new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer J. 3, e143 (2013).
Dimopoulos, M. et al. Pomalidomide, bortezomib, and dexamethasone for multiple myeloma previously treated with lenalidomide (OPTIMISMM): outcomes by prior treatment at first relapse. Leukemia 35, 1722–1731 (2021).
Swami, A. et al. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc. Natl Acad. Sci. USA 111, 10287–10292 (2014).
Ashley, J. D. et al. Liposomal bortezomib nanoparticles via boronic ester prodrug formulation for improved therapeutic efficacy in vivo. J. Med. Chem. 57, 5282–5292 (2014).
Xu, W. et al. Acid-labile boronate-bridged dextran–bortezomib conjugate with up-regulated hypoxic tumor suppression. Chem. Commun. 51, 6812–6815 (2015).
Lu, X. et al. Bortezomib dendrimer prodrug‐based nanoparticle system. Adv. Funct. Mater. 29, 1807941 (2019).
Zhu, J. et al. Bortezomib-catechol conjugated prodrug micelles: combining bone targeting and aryl boronate-based pH-responsive drug release for cancer bone-metastasis therapy. Nanoscale 10, 18387–18397 (2018).
Detappe, A., Bustoros, M., Mouhieddine, T. H. & Ghoroghchian, P. P. Advancements in nanomedicine for multiple myeloma. Trends Mol. Med. 24, 560–574 (2018).
Mu, C.-F. et al. Targeted drug delivery for tumor therapy inside the bone marrow. Biomaterials 155, 191–202 (2018).
Zhong, W., Zhang, X., Zhao, M., Wu, J. & Lin, D. Advancements in nanotechnology for the diagnosis and treatment of multiple myeloma. Biomater. Sci. 8, 4692–4711 (2020).
Ashley, J. D. et al. Dual carfilzomib and doxorubicin–loaded liposomal nanoparticles for synergistic efficacy in multiple myeloma. Mol. Cancer Ther. 15, 1452–1459 (2016).
Soodgupta, D. et al. Small molecule MYC inhibitor conjugated to integrin-targeted nanoparticles extends survival in a mouse model of disseminated multiple myeloma. Mol. Cancer Ther. 14, 1286–1294 (2015).
Deshantri, A. K. et al. Complete tumor regression by liposomal bortezomib in a humanized mouse model of multiple myeloma. Hemasphere 4, e463 (2020).
Deshantri, A. K. et al. Liposomal dexamethasone inhibits tumor growth in an advanced human-mouse hybrid model of multiple myeloma. J. Control. Release 296, 232–240 (2019).
Nguyen, H. V.-T. et al. Scalable synthesis of multivalent macromonomers for ROMP. ACS Macro Lett. 7, 472–476 (2018).
Liu, J. et al. ‘Brush-first’ method for the parallel synthesis of photocleavable, nitroxide-labeled PEG star polymers. J. Am. Chem. Soc. 134, 16337–16344 (2012).
Sowers, M. A. et al. Redox-responsive branched-bottlebrush polymers for in vivo MRI and fluorescence imaging. Nat. Commun. 5, 5460 (2014).
Stubelius, A., Lee, S. & Almutairi, A. The chemistry of boronic acids in nanomaterials for drug delivery. Acc. Chem. Res. 52, 3108–3119 (2019).
Antonio, J. P. M., Russo, R., Carvalho, C. P., Cal, P. M. S. D. & Gois, P. M. P. Boronic acids as building blocks for the construction of therapeutically useful bioconjugates. Chem. Soc. Rev. 48, 3513–3536 (2019).
Brooks, W. L. A. & Sumerlin, B. S. Synthesis and applications of boronic acid-containing polymers: from materials to medicine. Chem. Rev. 116, 1375–1397 (2016).
Graham, B. J., Windsor, I. W., Gold, B. & Raines, R. T. Boronic acid with high oxidative stability and utility in biological contexts. Proc. Natl Acad. Sci. USA 118, e2013691118 (2021).
Millennium Pharmaceuticals, Inc. Approval Package for Application Number 21-602/S-015 (Velcade). Center for Drug Evaluation and Research (2008).
Merz, M. et al. Subcutaneous versus intravenous bortezomib in two different induction therapies for newly diagnosed multiple myeloma: an interim analysis from the prospective GMMG-MM5 trial. Haematologica 100, 964–969 (2015).
Fink, E. C. et al. CrbnI391V is sufficient to confer in vivo sensitivity to thalidomide and its derivatives in mice. Blood 132, 1535–1544 (2018).
Hemeryck, A. et al. Tissue distribution and depletion kinetics of bortezomib and bortezomib-related radioactivity in male rats after single and repeated intravenous injection of 14C-bortezomib. Cancer Chemother. Pharmacol. 60, 777–787 (2007).
Sanchorawala, V. et al. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood 130, 597–605 (2017).
Summers, H. D. et al. Statistical analysis of nanoparticle dosing in a dynamic cellular system. Nat. Nanotechnol. 6, 170–174 (2011).
Rees, P., Wills, J. W., Brown, M. R., Barnes, C. M. & Summers, H. D. The origin of heterogeneous nanoparticle uptake by cells. Nat. Commun. 10, 2341 (2019).
Lancet, J. E. et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J. Clin. Oncol. 36, 2684–2692 (2018).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Acknowledgements
We thank the NIH-NCI (1R01CA220468-01 (J.A.J., P.P.G.) and R01CA205954 (I.M.G.)), the Leukemia and Lymphoma Society and the National Science Foundation (Graduate Research Fellowship (H.V.-T.N.)) for supporting this research. This work was further supported in part by the Koch Institute Core Grant P30-CA14051 from the NCI. A.D. acknowledges support from the International Myeloma Foundation, the Fondation Française pour la Recherche contre le Myélome et les Gammapathies (FFRMG) and Inserm Cancer. A.D., J.A.J. and I.M.G. acknowledge support from the Stand Up to Cancer Dream Team Multiple Myeloma grant. P.P.G. acknowledges the generous support of the Charles W. and Jennifer C. Johnson Clinical Investigator Fund as well as the Kathryn Fox Samway Foundation.
Author information
Authors and Affiliations
Contributions
J.A.J., P.P.G., I.M.G., A.D. and H.V.-T.N. conceived the project idea. H.V.-T.N., Y.J., W.W. and S.L.K. synthesized and characterized the materials. A.D., M.P.A. and C.M. performed the biological experiments. D.J.L. performed the computational simulations. S.B. conducted the cryogenic electron microscopy studies. All authors helped in analysing the results. J.A.J., P.P.G., I.M.G., A.D. and H.V.-T.N. wrote the paper. All authors read and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A.D., H.V.-T.N., Y.J., I.M.G., P.P.G. and J.A.J. are named inventors on a patent application (US patent application no. 16/825,269) jointly filed by the Massachusetts Institute of Technology and the Dana-Farber Cancer Institute on the Btz macromolecular PIs described in this work. H.V.-T.N., Y.J. and J.A.J. are co-founders and shareholders of Window Therapeutics, which seeks to translate this technology to clinical cancer therapies. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Jo Caers, Twan Lammers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–37, Tables 1 and 2, materials/general methods/instrumentation and synthesis procedures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Detappe, A., Nguyen, H.VT., Jiang, Y. et al. Molecular bottlebrush prodrugs as mono- and triplex combination therapies for multiple myeloma. Nat. Nanotechnol. 18, 184–192 (2023). https://doi.org/10.1038/s41565-022-01310-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01310-1
This article is cited by
-
Multidrug nanomedicine
Nature Nanotechnology (2023)
-
Bottlebrush prodrug provides a novel strategy for nanomedicine-mediated combination therapy of multiple myeloma
Science China Materials (2023)