Abstract
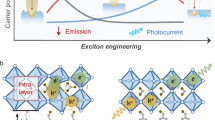
Molecular packing controls optoelectronic properties in organic molecular nanomaterials. Here we report a donor–acceptor organic molecule (2,6-bis(4-cyanophenyl)-4-(9-phenyl-9H-carbazol-3-yl)pyridine-3,5-dicarbonitrile) that exhibits two aggregate states in aqueous dispersions: amorphous nanospheres and ordered nanofibres with π–π molecular stacking. The nanofibres promote sacrificial photocatalytic H2 production (31.85 mmol g−1 h−1) while the nanospheres produce hydrogen peroxide (H2O2) (3.20 mmol g−1 h−1 in the presence of O2). This is the first example of an organic photocatalyst that can be directed to produce these two different solar fuels simply by changing the molecular packing. These different packings affect energy band levels, the extent of excited state delocalization, the excited state dynamics, charge transfer to O2 and the light absorption profile. We use a combination of structural and photophysical measurements to understand how this influences photocatalytic selectivity. This illustrates the potential to achieve multiple photocatalytic functionalities with a single organic molecule by engineering nanomorphology and solid-state packing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated and analysed relevant to the study are included in the Article and its Supplementary Information files. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2157381 and 2157384 for CNP-C1 and CNP-C2, respectively. These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
References
Weingarten, A. S. et al. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat. Chem. 6, 964–970 (2014).
Wagner, A., Sahm, C. D. & Reisner, E. Towards molecular understanding of local chemical environment effects in electro- and photocatalytic CO2 reduction. Nat. Catal. 3, 775–786 (2020).
Liu, D., Wang, J., Bai, X., Zong, R. & Zhu, Y. Self-assembled PDINH supramolecular system for photocatalysis under visible light. Adv. Mater. 28, 7284–7290 (2016).
Shigemitsu, H. et al. Aggregation-induced photocatalytic activity and efficient photocatalytic hydrogen evolution of amphiphilic rhodamines in water. Chem. Sci. 11, 11843–11848 (2020).
Giri, G. et al. Tuning charge transport in solution-sheared organic semiconductors using lattice strain. Nature 480, 504–508 (2011).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Tantakitti, F. et al. Energy landscapes and functions of supramolecular systems. Nat. Mater. 15, 469–476 (2016).
Li, Q. & Li, Z. Molecular packing: another key point for the performance of organic and polymeric optoelectronic materials. Acc. Chem. Res. 53, 962–973 (2020).
Rivnay, J. et al. Large modulation of carrier transport by grain-boundary molecular packing and microstructure in organic thin films. Nat. Mater. 8, 952–958 (2009).
Mutai, T., Satou, H. & Araki, K. Reproducible on–off switching of solid-state luminescence by controlling molecular packing through heat-mode interconversion. Nat. Mater. 4, 685–687 (2005).
Coropceanu, V. et al. Charge transport in organic semiconductors. Chem. Rev. 107, 926–952 (2007).
Kazantsev, R. V. et al. Crystal-phase transitions and photocatalysis in supramolecular scaffolds. J. Am. Chem. Soc. 139, 6120–6127 (2017).
McDowall, D. et al. Controlling photocatalytic activity by self-assembly—tuning perylene bisimide photocatalysts for the hydrogen evolution reaction. Adv. Energy Mater. 10, 2002469 (2020).
Weingarten, A. S. et al. Supramolecular packing controls H2 photocatalysis in chromophore amphiphile hydrogels. J. Am. Chem. Soc. 137, 15241–15246 (2015).
Korevaar, P. A. et al. Pathway complexity in supramolecular polymerization. Nature 481, 492–496 (2012).
Ogi, S., Sugiyasu, K., Manna, S., Samitsu, S. & Takeuchi, M. Living supramolecular polymerization realized through a biomimetic approach. Nat. Chem. 6, 188–195 (2014).
Kudo, A. & Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009).
Zhang, G., Lan, Z. A. & Wang, X. Conjugated polymers: catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 55, 15712–15727 (2016).
Hou, H., Zeng, X. & Zhang, X. Production of hydrogen peroxide by photocatalytic processes. Angew. Chem. Int. Ed. 59, 17356–17376 (2020).
Kosco, J. et al. Enhanced photocatalytic hydrogen evolution from organic semiconductor heterojunction nanoparticles. Nat. Mater. 19, 559–565 (2020).
Shiraishi, Y. et al. Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion. Nat. Mater. 18, 985–993 (2019).
Lin, L. et al. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 3, 649–655 (2020).
Benniston, A. C., Harriman, A., Lawrie, D. J. & Mayeux, A. The photophysical properties of a pyrene–thiophene–terpyridine conjugate and of its zinc(II) and ruthenium(II) complexes. Phys. Chem. Chem. Phys. 6, 51–57 (2004).
Zhou, C. et al. Enhancing the electroluminescent efficiency of acridine-based donor–acceptor materials: quasi-equivalent hybridized local and charge-transfer state. J. Phys. Chem. C 122, 18376–18382 (2018).
Dhara, A. et al. Zero-overlap fluorophores for fluorescent studies at any concentration. J. Am. Chem. Soc. 142, 12167–12180 (2020).
Guo, Y., Shi, W. & Zhu, Y. Internal electric field engineering for steering photogenerated charge separation and enhancing photoactivity. EcoMat 1, 1–20 (2019).
Schubert, S., Delaney, J. T. & Schubert, U. S. Nanoprecipitation and nanoformulation of polymers: from history to powerful possibilities beyond poly(lactic acid). Soft Matter 7, 1581–1588 (2011).
Ramírez, C. L., Mangione, M. I., Bertolotti, S. G., Arbeloa, E. M. & Parise, A. R. A photophysical and spectroelectrochemical study on N-phenyl-carbazoles and their oxidized species. J. Photochem. Photobiol. A Chem. 365, 199–207 (2018).
Neelambra, A. U., Govind, C., Devassia, T. T., Somashekharappa, G. M. & Karunakaran, V. Direct evidence of solvent polarity governing the intramolecular charge and energy transfer: ultrafast relaxation dynamics of push–pull fluorene derivatives. Phys. Chem. Chem. Phys. 21, 11087–11102 (2019).
Wang, X. et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 10, 1180–1189 (2018).
Maeda, K. et al. Photocatalytic activities of graphitic carbon nitride powder for water reduction and oxidation under visible light. J. Phys. Chem. C. 113, 4940–4947 (2009).
Liu, L. et al. Linear conjugated polymers for solar-driven hydrogen peroxide production: the importance of catalyst stability. J. Am. Chem. Soc. 143, 19287–19293 (2021).
CrysAlisPRO, 2020 version (Oxford Diffraction/Agilent Technologies UK, 2020).
Sheldrick, G. M. A short history of SHELX. Acta Cryst. A6 4, 112–122 (2008).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Cryst. C7 1, 3–8 (2015).
Nowell, H., Barnett, S. A., Christensen, K. E., Teat, S. J. & Allan, D. R. I19, the small-molecule single-crystal diffraction beamline at Diamond Light Source. J. Synchrotron Radiat. 19, 435–441 (2012).
Allan, D. et al. Novel dual air-bearing fixed-χ diffractometer for small-molecule single-crystal X-ray diffraction on beamline I19 at Diamond Light Source. Crystals 7, 336 (2017).
Coelho, A. A. TOPAS and TOPAS-Academic: an optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Cryst. 51, 210–218 (2018).
Dollase, W. A. Correction of intensities for preferred orientation in powder diffractometry: application of the March model. J. Appl. Cryst. 19, 267–272 (1986).
Wei, Z. et al. Efficient visible-light-driven selective oxygen reduction to hydrogen peroxide by oxygen-enriched graphitic carbon nitride polymers. Energy Environ. Sci. 11, 2581–2589 (2018).
Yanai, T., Tew, D. P. & Handy, N. C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Liu, Z., Lu, T. & Chen, Q. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: electronic structure, electronic spectrum, and optical nonlinearity. Carbon 165, 461–467 (2020).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian, 2016).
Bannwarth, C. & Grimme, S. A simplified time-dependent density functional theory approach for electronic ultraviolet and circular dichroism spectra of very large molecules. Comput. Theor. Chem. 1040, 45–53 (2014).
Hanwell, M. D. et al. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 4, 1–17 (2012).
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EPSRC) for financial support under grants EP/N004884/1 and EP/P034497/1. TA measurements were performed at the University of Liverpool Early Career Researcher Laser Laboratory supported by UKRI-EPSRC grant EP/S017623/1 and the University of Liverpool, maintained and operated as a shared research facility by the Faculty of Science and Engineering. We are grateful to B. Greeves (University of Liverpool) for assistance with performing the spectroelectrochemical measurements and M. Volk for discussion on 1O2. H.Y. thanks the Leverhulme Trust via the Leverhulme Research Centre for Functional Materials Design for funding. H.Y. thanks M. Little for the input on the crystal measurements and discussion. C.L. thanks the China Scholarship Council for a PhD studentship. Weiwei Zhang acknowledges support from the Fundamental Research Funds for the Central Universities and Shanghai Pujiang Program (22PJ1402400). The TEM experiments in this paper were performed in the Albert Crewe Centre for Electron Microscopy at the University of Liverpool, maintained and operated as a shared research facility by the Faculty of Science and Engineering. Computing support from the High Performance Computing facility at the University of Liverpool is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
H.Y. synthesized and characterized CNP nanoparticles, grew the crystals, and performed photocatalysis experiments and analysis. C.L. carried out TA, Raman experiments, spectroelectrochemistry, singlet oxygen measurements and spectra analysis with help from A.M.G. and A.J.C. T.L. performed DFT calculations. T.F. and L.C. measured the single crystal. S.Y.C. performed simulations on nanoparticle structures. L.L. helped with electrochemical characterization. Weiwei Zhang and Y.X. were involved in the analysis of CNP photoactivity. Wei Zhao helped with isotopic exchange experiments. M.B. and N.D.B carried out the HRTEM measurements. R.C. helped with the instrument build. X.L. synthesized the CNP molecules and performed the initial study. X.L., A.J.C. and A.I.C. supervised this work. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Single crystal structure of CNP.
a, Single crystal structure of the P212121 CNP phase grown by sublimation at 683 K under dynamic vacuum (grey, C; blue, N; write, H). b, The experimental X-ray diffraction pattern of CNP-C1. c, Single crystal structure of CNP-C2.
Extended Data Fig. 2 Transient absorption spectra of small molecule in THF.
a,b,c, Transient absorption spectra of donor (a), acceptor (b), and CNP molecule (c) (100 μM) in THF following 325 nm excitation with a power of 750 µW, respectively. d,e,f, Transient absorption spectra of donor (d), acceptor (e), and CNP molecule (f) (100 μM) in THF following 380 nm excitation with a power of 750 µW, respectively. All the samples are under an argon gas atmosphere in a 1 mm pathlength cuvette.
Extended Data Fig. 3 Cyclic voltammogram (CV) and spectroelectrochemical spectra of molecules in acetonitrile.
a, Cyclic voltammogram (CV) of CNP, donor, acceptor in acetonitrile recorded at 0.1 V s−1 from −2.6 V to +1.4 V vs Fc+/Fc. b-f, Spectroelectrochemical spectra in acetonitrile upon (1) stepwise increase of the potential from 0 to 1.2 V (CNP (b), acceptor (d)) and 0 to 1.4 V (donor (f)) and (2) stepwise decrease of the potential from 0 to -2.2 V (CNP (c), acceptor (e)) and 0 to -2 V (donor (g)), which are calibrated versus the ferrocene/ferrocenium redox couple.
Extended Data Fig. 4 Isotope labelling experiments.
a, Time course of the gas production of CNP-f (2.5 mg) in a D2O solution containing 0.1 M ascorbic acid (20 mL). The reactions were carried out under visible illumination (300 W Xe light source, λ > 420 nm) with side-illumination through a quartz window. Percentage of gas products were measured by mass spectrometry. b, Isotopic 18O2 labelling experiments with 18O2 in photocatalytic H2O2 production irradiated by 300 W Xe lamp fitted with a λ > 420 nm filter using 50 mg of the CNP-s in 18O2 environment. The signal of 16O2 was from air during GC-MS injection.
Extended Data Fig. 5 Isotope labelling experiments.
Transient absorption spectra and normalized single wavelength dynamics at the charge transfer states around 840–860 nm in the presence of 100 mM AA (CNP-s (a), CNP-f (c)) and the mixture of 100 mM AA and 3 wt% Pt (CNP-s (b), CNP-f (d)) in water on the fs–ns timescale.
Supplementary information
Supplementary information
Supplementary Figs. 1–44, Discussion and Tables 1–6.
Source data
Source Data Fig. 1
Source Data Fig. 1
Source Data Fig. 2
Source Data Fig. 2
Source Data Fig. 3
Source Data Fig. 3
Source Data Fig. 4
Source Data Fig. 4
Source Data Fig. 5
Source Data Fig. 5
Source Data Extended Data Fig. 1
SourceData_ExtendedData_Fig1
Source Data Extended Data Fig. 2
SourceData_ExtendedData_Fig2
Source Data Extended Data Fig. 3
SourceData_ExtendedData_Fig3
Source Data Extended Data Fig. 4
SourceData_ExtendedData_Fig4
Source Data Extended Data Fig. 5
SourceData_ExtendedData_Fig5
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Li, C., Liu, T. et al. Packing-induced selectivity switching in molecular nanoparticle photocatalysts for hydrogen and hydrogen peroxide production. Nat. Nanotechnol. 18, 307–315 (2023). https://doi.org/10.1038/s41565-022-01289-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01289-9
This article is cited by
-
Photocatalytic aerobic oxidation of C(sp3)-H bonds
Nature Communications (2024)
-
Accelerated discovery of molecular nanojunction photocatalysts for hydrogen evolution by using automated screening and flow synthesis
Nature Synthesis (2024)
-
Carbon quantum dots-modified tetra (4-carboxyphenyl) porphyrin/BiOBr S-scheme heterojunction for efficient photocatalytic antibiotic degradation
Science China Materials (2024)
-
Research with robotics
Nature Synthesis (2023)