Abstract
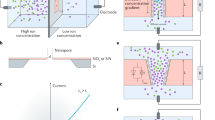
Osmotic power, also known as ‘blue energy’, is produced by mixing solutions of different salt concentrations, and represents a vast, sustainable and clean energy source. The efficiency of harvesting osmotic power is primarily determined by the transmembrane performance, which is in turn dependent on ion conductivity and selectivity towards positive or negative ions. Atomically or molecularly thin membranes with a uniform pore environment and high pore density are expected to possess an outstanding ion permeability and selectivity, but remain unexplored. Here we demonstrate that covalent organic framework monolayer membranes that feature a well-ordered pore arrangement can achieve an extremely low membrane resistivity and ultrahigh ion conductivity. When used as osmotic power generators, these membranes produce an unprecedented output power density over 200 W m−2 on mixing the artificial seawater and river water. This work opens up the application of porous monolayer membranes with an atomically precise structure in osmotic power generation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data that support the plots within this paper and other findings of this study are available from the corresponding author on reasonable request.
References
Pattle, R. E. Production of electric power by mixing fresh and salt water in the hydroelectric pile. Nature 174, 660–660 (1954).
Logan, B. E. & Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 488, 313–319 (2012).
Siria, A., Bocquet, M. L. & Bocquet, L. New avenues for the large-scale harvesting of blue energy. Nat. Rev. Chem. 1, 0091 (2017).
Park, H. B., Kamcev, J., Robeson, L. M., Elimelech, M. & Freeman, B. D. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017).
Xiao, K., Jiang, L. & Antonietti, M. Ion transport in nanofluidic devices for energy harvesting. Joule 3, 2364–2380 (2019).
Zhang, Z., Wen, L. & Jiang, L. Nanofluidics for osmotic energy conversion. Nat. Rev. Mater. https://doi.org/10.1038/s41578-021-00300-4 (2021).
Shen, J., Liu, G. P., Han, Y. & Jin, W. Q. Artificial channels for confined mass transport at the sub-nanometre scale. Nat. Rev. Mater. 6, 294–312 (2021).
Macha, M., Marion, S., Nandigana, V. V. R. & Radenovic, A. 2D materials as an emerging platform for nanopore-based power generation. Nat. Rev. Mater. 4, 588–605 (2019).
Rollings, R. C., Kuan, A. T. & Golovchenko, J. A. Ion selectivity of graphene nanopores. Nat. Commun. 7, 11408 (2016).
Bocquet, L. Nanofluidics coming of age. Nat. Mater. 19, 254–256 (2020).
Feng, J. D. et al. Single-layer MoS2 nanopores as nanopower generators. Nature 536, 197–200 (2016).
Liu, X. et al. Power generation by reverse electrodialysis in a single-layer nanoporous membrane made from core–rim polycyclic aromatic hydrocarbons. Nat. Nanotechnol. 15, 307–312 (2020).
Wang, H. et al. Blue energy conversion from holey-graphene-like membranes with a high density of subnanometer pores. Nano Lett. 20, 8634–8639 (2020).
Surwade, S. P. et al. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 10, 459–464 (2015).
Graf, M. et al. Fabrication and practical applications of molybdenum disulfide nanopores. Nat. Protocols 14, 1130–1168 (2019).
Cote, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Geng, K. Y. et al. Covalent organic frameworks: design, synthesis, and functions. Chem. Rev. 120, 8814–8933 (2020).
Yuan, S. S. et al. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 48, 2665–2681 (2019).
Lohse, M. S. & Bein, T. Covalent organic frameworks: structures, synthesis, and applications. Adv. Funct. Mater. 28, 1705553 (2018).
Zhong, Y. et al. Wafer-scale synthesis of monolayer two-dimensional porphyrin polymers for hybrid superlattices. Science 366, 1379–1384 (2019).
Sahabudeen, H. et al. Wafer-sized multifunctional polyimine-based two-dimensional conjugated polymers with high mechanical stiffness. Nat. Commun. 7, 13461 (2016).
Wang, L. et al. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nat. Nanotechnol. 12, 509–522 (2017).
Qi, H. Y. et al. Near-atomic-scale observation of grain boundaries in a layer-stacked two-dimensional polymer. Sci. Adv. 6, eabb5976 (2020).
Stein, D., Kruithof, M. & Dekker, C. Surface-charge-governed ion transport in nanofluidic channels. Phys. Rev. Lett. 93, 035901 (2004).
Duan, C. H. & Majumdar, A. Anomalous ion transport in 2-nm hydrophilic nanochannels. Nat. Nanotechnol. 5, 848–852 (2010).
Siwy, Z., Heins, E., Harrell, C. C., Kohli, P. & Martin, C. R. Conical-nanotube ion-current rectifiers: the role of surface charge. J. Am. Chem. Soc. 126, 10850–10851 (2004).
Walker, M. I. et al. Extrinsic cation selectivity of 2D membranes. ACS Nano 11, 1340–1346 (2017).
Wang, L., Wang, Z., Patel, S. K., Lin, S. & Elimelech, M. Nanopore-based power generation from salinity gradient: why it is not viable. ACS Nano 15, 4093–4107 (2021).
Green, Y., Park, S. & Yossifon, G. Bridging the gap between an isolated nanochannel and a communicating multipore heterogeneous membrane. Phys. Rev. E 91, 011002 (2015).
Xiao, F. L. et al. A general strategy to simulate osmotic energy conversion in multi-pore nanofluidic systems. Mater. Chem. Front. 2, 935–941 (2018).
Tsutsui, M. et al. Electric field interference and bimodal particle translocation in nano-integrated multipores. Nanoscale 11, 7547–7553 (2019).
Vlassiouk, I., Smirnov, S. & Siwy, Z. Ionic selectivity of single nanochannels. Nano Lett. 8, 1978–1985 (2008).
Fu, Y. J., Guo, X., Wang, Y. H., Wang, X. W. & Xue, J. M. An atomically-thin graphene reverse electrodialysis system for efficient energy harvesting from salinity gradient. Nano Energy 57, 783–790 (2019).
Chen, J. J. et al. Ultrathin and robust silk fibroin membrane for high-performance osmotic energy conversion. ACS Energy Lett. 5, 742–748 (2020).
Zhang, Z. et al. Engineered asymmetric heterogeneous membrane: a concentration-gradient-driven energy harvesting device. J. Am. Chem. Soc. 137, 14765–14772 (2015).
Yan, F., Yao, L. N., Chen, K. X., Yang, Q. & Su, B. An ultrathin and highly porous silica nanochannel membrane: toward highly efficient salinity energy conversion. J. Mater. Chem. A 7, 2385–2391 (2019).
Gao, J. et al. High-performance ionic diode membrane for salinity gradient power generation. J. Am. Chem. Soc. 136, 12265–12272 (2014).
Hong, S. et al. Two-dimensional Ti3C2Tx MXene membranes as nanofluidic osmotic power generators. ACS Nano 13, 8917–8925 (2019).
Zhu, X. B. et al. Unique ion rectification in hypersaline environment: a high-performance and sustainable power generator system. Sci. Adv. 4, eaau1665 (2018).
Li, R. R., Jiang, J. Q., Liu, Q. Q., Xie, Z. Q. & Zhai, J. Hybrid nanochannel membrane based on polymer/MOF for high-performance salinity gradient power generation. Nano Energy 53, 643–649 (2018).
Kwak, S. H. et al. Densely charged polyelectrolyte-stuffed nanochannel arrays for power generation from salinity gradient. Sci. Rep. 6, 26416 (2016).
Hou, S. H. et al. Free-standing covalent organic framework membrane for high-efficiency salinity gradient energy conversion. Angew. Chem. Int. Ed. 60, 9925–9930 (2021).
Li, C. et al. Large-scale, robust mushroom-shaped nanochannel array membrane for ultrahigh osmotic energy conversion. Sci. Adv. 7, eabg2183 (2021).
Vermaas, D. A., Veerman, J., Saakes, M. & Nijmeijer, K. Influence of multivalent ions on renewable energy generation in reverse electrodialysis. Energ. Environ. Sci. 7, 1434–1445 (2014).
Schaetzle, O. & Buisman, C. J. N. Salinity gradient energy: current state and new trends. Engineering 1, 164–166 (2015).
Yao, Y. et al. High performance polyester reverse osmosis desalination membrane with chlorine resistance. Nat. Sustain. 4, 138–146 (2021).
Matsumoto, M. et al. Rapid, low temperature formation of imine-linked covalent organic frameworks catalyzed by metal triflates. J. Am. Chem. Soc. 139, 4999–5002 (2017).
Nečas, D. & Klapetek, P. Gwyddion: an open-source software for SPM data analysis. Cent. Eur. J. Phys. 10, 181–188 (2012).
Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 113, 7756–7764 (2000).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Acknowledgements
We acknowledge financial support from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB36000000, Z.T.), National Key Basic Research Program of China (2016YFA0200700, Z.T.), National Natural Science Foundation of China (21922504 and 52073069, L.L., and 92056204, 21890381 and 21721002, Z.T.), Frontier Science Key Project of the Chinese Academy of Sciences (QYZDJ-SSW-SLH038, Z.T.), Youth Innovation Promotion Association CAS (2018046, L.L.), China National Postdoctoral Program for Innovative Talents (BX20200102, J.Y.) and China Postdoctoral Science Foundation (2019M660583, J.Y.). K. Peng is acknowledged for focused ion beam (FIB) technical help.
Author information
Authors and Affiliations
Contributions
L.L., J.Y. and Z.T. conceived the research idea, designed all the experiments and wrote the manuscript. J.Y., P.L. and M.F. carried out the experimental measurements and data analysis with help from K.H., J.W. and Z.Y. B.T. performed the simulations and interpreted the simulation results. G.Z., Z.H., J.H., Q.F. and X.Q. joined the discussion of the data and gave useful suggestions. All the authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Soryong Chae and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Electrochemical measurement.
a, Scheme of the experimental setup. ZnTPP-COF monolayer membrane suspending on the SiNx/Si chip containing a 2 μm aperture is mounted on a Teflon disk, with both sides of the membrane in contact with aqueous solution. Ag/AgCl electrodes are applied to characterize the current-voltage response, where agarose salt bridges are used to eliminate the imbalanced redox potential at the electrode|electrolyte interface. b, Photograph of the disassembled electrochemical device. c, An empty SiNx aperture and a ZnTPP-COF monolayer membrane covered SiNx aperture.
Extended Data Fig. 2 Ion selectivity simulation.
a, The simulated 2D domain for PNP model. The pore length and diameter of each pore is 0.6 nm and 2 nm, respectively. In all the simulations, the concentration and potential of the central pore are used to calculate the current. b, c, Calculated steady-state concentration distribution of K+ (b) and Cl− (c) near ZnTPP-COF monolayer membrane with a surface charge density of 4.1 mC m-2 under a salt gradient of 10 mM/0.1 mM, respectively. The higher concentration of Cl− (counter-ions) compared with that of K+ (co-ions) in the whole pore region suggests the anion selectivity of the membrane. d, An electrostatic potential under salt gradient of 10 mM/0.1 mM builds up along the salinity gradient direction, owing to the selective transport of anions across the membrane.
Extended Data Fig. 3 Investigation on the pore-pore coupling effect.
a, Electrostatic potential showing different pore-pore coupling between the central pore and the 1-1, 2-2, 3-3, and 4-4 pores. b, Ion selectivity of the central pore upon coupling with the 1-1, 2-2, 3-3, and 4-4 pores. c, Osmotic current of the central pore upon coupling with the 1-1, 2-2, 3-3, and 4-4 pores. Surface charge density is set to be 4.1 mC m−2 under a salt gradient of 10 mM KCl/0.1 mM KCl.
Extended Data Fig. 4 Effect of interpore distance on the pore-pore coupling effect.
a, Electrostatic potential built under varied pore distance of 2.5 nm to 8.5 nm. b, Ion selectivity of the central pore when the interpore distance increases from 2.5 nm to 8.5 nm. c, Osmotic current of the central pore when the interpore distance increases from 2.5 nm to 8.5 nm. Surface charge density is set to be 4.1 mC m−2 under a salt gradient of 10 mM KCl/0.1 mM KCl. In the range of the interpore distance larger than 4.5 nm, small change in ion selectivity but obvious drop in osmotic current are discerned with the interpore distance decreasing (in other words, the pore density increasing), which reflects the effect of enhanced concentration polarization or ion transport resistance. On the contrary, in the range of the interpore distance smaller than 4.5 nm, a positive gain of pore-pore coupling effect comes to play a dominant role, namely, large increase in ion selectivity. This result reveals big enhancement of ion selectivity due to the strong pore-pore coupling. Meanwhile, the enhancement of osmotic current caused by the pore-pore coupling effect greatly compensates the decrease of osmotic current caused by concentration polarization or ion transport resistance, as the interpore distance decreases to below 4.5 nm (Extended Data Fig. 4c).
Extended Data Fig. 5 Atomic charge distribution of ZnTPP-COF (a), NiTPP-COF (b) and CuTPP-COF (c) from DFT calculation.
The metal centers show positive charge intensity in the order of ZnTPP-COF > NiTPP-COF > CuTPP-COF, which is consistent with the experimental trend on the positive surface charge density of these membranes. (orange: Zn, teal: Ni, green: Cu, blue: N, red: O, grey: C, white: H).
Extended Data Fig. 6 Osmotic power generation by NiTPP-COF and CuTPP-COF monolayer membranes.
Current-voltage curves (a, d) as well as current density and output power density on the external load resistance (b, e) of NiTPP-COF (a, b) and CuTPP-COF (d, e) monolayer membranes in the artificial seawater/artificial river water gradient system. Ios and Vos are the osmotic current and voltage, respectively. The current density-time curves of NiTPP-COF (c) and CuTPP-COF (f) monolayer membranes with electrolyte replenishing every 50 min operated under the condition of the maximum output power density.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23, discussion and Tables 1–5.
Rights and permissions
About this article
Cite this article
Yang, J., Tu, B., Zhang, G. et al. Advancing osmotic power generation by covalent organic framework monolayer. Nat. Nanotechnol. 17, 622–628 (2022). https://doi.org/10.1038/s41565-022-01110-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01110-7
This article is cited by
-
Electricity generation from carbon dioxide adsorption by spatially nanoconfined ion separation
Nature Communications (2024)
-
Bioinspired light-driven chloride pump with helical porphyrin channels
Nature Communications (2024)
-
Unlocking osmotic energy harvesting potential in challenging real-world hypersaline environments through vermiculite-based hetero-nanochannels
Nature Communications (2024)
-
Light-responsive and ultrapermeable two-dimensional metal-organic framework membrane for efficient ionic energy harvesting
Nature Communications (2024)
-
Efficient osmosis-powered production of green hydrogen
Nature Sustainability (2024)