Abstract
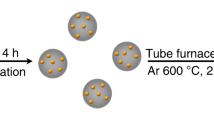
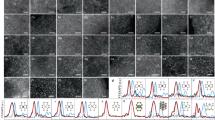
Single-atom catalysts have recently attracted considerable attention because of their highly efficient metal utilization and unique properties. Finding a green, facile method to synthesize them is key to their widespread commercialization. Here we show that single-atom catalysts (including iron, cobalt, nickel and copper) can be prepared via a top-down abrasion method, in which the bulk metal is directly atomized onto different supports, such as carbon frameworks, oxides and nitrides. The level of metal loading can be easily tuned by changing the abrasion rate. No synthetic chemicals, solvents or even water were used in the process and no by-products or waste were generated. The underlying reaction mechanism involves the mechanochemical force in situ generating defects on the supports, then trapping and stably sequestering atomized metals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are presented in the main text and the Supplementary Information, and are available from the corresponding authors upon reasonable request.
References
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Cui, X., Li, W., Ryabchuk, P., Junge, K. & Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 1, 385–397 (2018).
Beniya, A. & Higashi, S. Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2, 590–602 (2019).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Therrien, A. J. et al. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 1, 192–198 (2018).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Li, H. et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 13, 411–417 (2018).
Yao, Y. et al. High temperature shockwave stabilized single atoms. Nat. Nanotechnol. 14, 851–857 (2019).
Malta, G. et al. Identification of single-site gold catalysis in acetylene hydrochlorination. Science 355, 1399–1403 (2017).
Chung, H. T. et al. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 357, 479–484 (2017).
Gu, J., Hsu, C. S., Bai, L., Chen, H. M. & Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 364, 1091–1094 (2019).
Zhang, J. et al. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 1, 985–992 (2018).
Liu, D. et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 4, 512–518 (2019).
Cao, L. et al. Identification of single-atom active sites in carbon-based cobalt catalysts during electrocatalytic hydrogen evolution. Nat. Catal. 2, 134–141 (2019).
Guan, J. et al. Water oxidation on a mononuclear manganese heterogeneous catalyst. Nat. Catal. 1, 870–877 (2018).
He, X. et al. Mechanochemical kilogram-scale synthesis of noble metal single-atom catalysts. Cell Rep. Phys. Sci. 1, 100004 (2020).
Jin, H. et al. Simple and scalable mechanochemical synthesis of noble metal catalysts with single atoms toward highly efficient hydrogen evolution. Adv. Funct. Mater. 30, 2000531 (2020).
Gan, T. et al. Unveiling the kilogram-scale gold single-atom catalysts via ball milling for preferential oxidation of CO in excess hydrogen. Chem. Eng. J. 389, 124490 (2020).
Gan, T. et al. Facile synthesis of kilogram-scale co-alloyed Pt single-atom catalysts via ball milling for hydrodeoxygenation of 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 8, 8692–8699 (2020).
Wei, S. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 13, 856–861 (2018).
Goodman, E. D. et al. Catalyst deactivation via decomposition into single atoms and the role of metal loading. Nat. Catal. 2, 748–755 (2019).
Qu, Y. et al. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat. Catal. 1, 781–786 (2018).
Han, G. F. et al. Dissociating stable nitrogen molecules under mild conditions by cyclic strain engineering. Sci. Adv. 5, eaax8275 (2019).
Wan, X. et al. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2, 259–268 (2019).
Zitolo, A. et al. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 14, 937–942 (2015).
Li, J. et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 1, 935–945 (2018).
Kramm, U. I., Lefèvre, M., Larouche, N., Schmeisser, D. & Dodelet, J. Correlations between mass activity and physicochemical properties of Fe/N/C catalysts for the ORR in PEM fuel cell via 57Fe Mössbauer spectroscopy and other techniques. J. Am. Chem. Soc. 136, 978–985 (2014).
Fei, H. et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018).
Tong, W. P., Tao, N. R., Wang, Z. B., Lu, J. & Lu, K. Nitriding iron at lower temperatures. Science 299, 686–688 (2003).
Cretu, O. et al. Migration and localization of metal atoms on strained graphene. Phys. Rev. Lett. 105, 196102 (2010).
Robertson, A. W. et al. Dynamics of single Fe atoms in graphene vacancies. Nano Lett. 13, 1468–1475 (2013).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Clark, S. J. et al. First principles methods using CASTEP. Z. Kristallogr. Cryst. Mater. 220, 567–570 (2005).
Acknowledgements
We thank M. Chhowalla for help and discussion. We are grateful for the use of the Pohang Accelerator Laboratory (6D UNIST-PAL beamline, South Korea). J.-B.B. acknowledges support from the Creative Research Initiative (CRI, 2014R1A3A2069102) and Science Research Center (SRC, 2016R1A5A1009405) programmes through the National Research Foundation (NRF) of Korea. J.-B.B also acknowledges the U-K Brand Project (1.200096.01) of UNIST. H.Y.J. acknowledges support from the National R&D Program (2020M3F3A2A01082618) through the National Research Foundation (NRF) of Korea. F.L. acknowledges financial support from Fudan University (JIH2203011). Funding for J.W., A.I.R., W.Z. and R.G. was provided by the International Partnership Program of the Chinese Academy of Sciences (121421KYSB20170020) and the State Key Laboratory of Catalysis in Dalian Institute of Chemical Physics (N-16-07). Z.A. acknowledges financial support (221777033) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Contributions
J.-B.B. conceived the project and oversaw all the research phases. J.-B.B. and G.-F.H. designed the project. G.-F.H. carried out the sample synthesis and structural characterization. F.L. and Z.A. performed the DFT calculation. T.J.S., Y.-K.I., J.-P.J. and S.-J.K. assisted with the XAS study. H.Y.J. conducted the HAADF-STEM measurements. J.W., A.I.R., W.Z. and R.G. measured and interpreted the Mössbauer spectroscopy. S.-Y.Y. designed the scheme. Data collection and analysis were conducted by J.-B.B., G.-F.H. and F.L. All the authors contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, notes, Figs. 1–15 and Tables 1–5.
Rights and permissions
About this article
Cite this article
Han, GF., Li, F., Rykov, A.I. et al. Abrading bulk metal into single atoms. Nat. Nanotechnol. 17, 403–407 (2022). https://doi.org/10.1038/s41565-022-01075-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01075-7
This article is cited by
-
Tailoring d-band center of high-valent metal-oxo species for pollutant removal via complete polymerization
Nature Communications (2024)
-
Spatial engineering of single-atom Fe adjacent to Cu-assisted nanozymes for biomimetic O2 activation
Nature Communications (2024)
-
Controlling tin whisker growth via oxygen-mediated decomposition of Ti2SnC
Journal of Materials Science (2024)
-
Asymmetrically coordinated main group atomic In-S1N3 interface sites for promoting electrochemical CO2 reduction
Nano Research (2024)
-
Efficient electrocatalytic reduction of CO2 to CO via mechanochemical synthesized copper-based composite metallic oxide catalyst
Journal of Porous Materials (2024)