Abstract
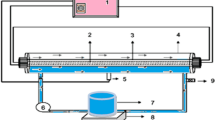
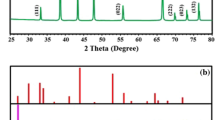
Micropollutants in the aquatic environment pose a high risk to both environmental and human health. The photocatalytic degradation of steroid hormones in a flow-through photocatalytic membrane reactor under UV light (365 nm) at environmentally relevant concentrations (50 ng l–1 to 1 mg l–1) was examined using a polyethersulfone–titanium dioxide (PES–TiO2) membrane. The TiO2 nanoparticles (10–30 nm) were immobilized both on the surface and in the nanopores (220 nm) of the membrane. Water quality and operational parameters were evaluated to elucidate the limiting factors in the degradation of steroid hormones. Flow through the photocatalytic membrane increased contact between the micropollutants and ·OH in the pores. Notably, 80% of both oestradiol and oestrone was removed from a 200 ng l–1 feed (at 25 mW cm–2 and 300 l m–2 h–1). Progesterone and testosterone removal was lower at 44% and 33%, respectively. Increasing the oestradiol concentration to 1 mg l–1 resulted in 20% removal, whereas with a 100 ng l–1 solution, a maximum removal of 94% was achieved at 44 mW cm–2 and 60 l m–2 h–1. The effectiveness of the relatively well-known PES–TiO2 membrane for micropollutant removal has been demonstrated; this effectiveness is due to the nanoscale size of the membrane, which provides a high surface area and facilitates close contact of the radicals with the very small (0.8 nm) micropollutant at an extremely low, environmentally relevant concentration (100 ng l–1).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that supports the findings of this research will be available upon request.
References
Escher, B. I., Stapleton, H. M. & Schymanski, E. L. Tracking complex mixtures of chemicals in our changing environment. Science 367, 388–392 (2020).
Johnson, A. C., Jin, X., Nakada, N. & Sumpter, J. P. Learning from the past and considering the future of chemicals in the environment. Science 367, 384–387 (2020).
Aemig, Q., Helias, A. & Patureau, D. Impact assessment of a large panel of organic and inorganic micropollutants released by wastewater treatment plants at the scale of France. Water Res. 188, 116524 (2021).
Gonzalez, A. et al. Steroid hormones and estrogenic activity in the wastewater outfall and receiving waters of the Chascomus chained shallow lakes system (Argentina). Sci. Total Environ. 743, 140401 (2020).
Adeel, M., Song, X., Wang, Y., Francis, D. & Yang, Y. Environmental impact of estrogens on human, animal and plant life: a critical review. Environ. Int. 99, 107–119 (2017).
Kuch, H. M. & Ballschmiter, K. Determination of endocrine-disrupting phenolic compounds and estrogens in surface and drinking water by HRGC−(NCI)−MS in the picogram per liter range. Environ. Sci. Technol. 35, 3201–3206 (2001).
Luo, Y. et al. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 473–474, 619–641 (2014).
Pelissero, C. et al. Vitellogenin synthesis in cultured hepatocytes; an in vitro test for the estrogenic potency of chemicals. J. Steroid Biochem. Mol. Biol. 44, 263–272 (1993).
Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December on the Quality of Water Intended for Human Consumption (EU, 2020).
Directive of the European Parliament and of the Council on the Quality of Water Intended for Human Consumption (Recast) 2017/0332(COD) (EU, 2018).
Alvarez, P. J. J., Chan, C. K., Elimelech, M., Halas, N. J. & Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotechnol. 13, 634–641 (2018).
Hodges, B. C., Cates, E. L. & Kim, J. H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 13, 642–650 (2018).
Mauter, M. S. et al. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 1, 166–175 (2018).
Fischer, K., Gläser, R. & Schulze, A. Nanoneedle and nanotubular titanium dioxide–PES mixed matrix membrane for photocatalysis. Appl. Catal. B 160–161, 456–464 (2014).
Ahmad, R. et al. Photocatalytic systems as an advanced environmental remediation: recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 4, 4143–4164 (2016).
Orozco-Hernández, L. et al. 17-β-Estradiol: significant reduction of its toxicity in water treated by photocatalysis. Sci. Total Environ. 669, 955–963 (2019).
Castellanos, R. M., Paulo Bassin, J., Dezotti, M., Boaventura, R. A. R. & Vilar, V. J. P. Tube-in-tube membrane reactor for heterogeneous TiO2 photocatalysis with radial addition of H2O2. Chem. Eng. J. 395, 124998 (2020).
Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 73, 71–91 (2010).
Wang, M. et al. Preliminary study on the removal of steroidal estrogens using TiO2-doped PVDF ultrafiltration membranes. Water 8, 134 (2016).
Tsehaye, M. T., Velizarov, S. & Van der Bruggen, B. Stability of polyethersulfone membranes to oxidative agents: a review. Polym. Degrad. Stab. 157, 15–33 (2018).
Berger, T. E., Regmi, C., Schäfer, A. I. & Richards, B. S. Photocatalytic degradation of organic dye via atomic layer deposited TiO2 on ceramic membranes in single-pass flow-through operation. J. Membr. Sci. 604, 118015 (2020).
Lyubimenko, R., Busko, D., Richards, B. S., Schäfer, A. I. & Turshatov, A. Efficient photocatalytic removal of methylene blue using a metalloporphyrin–poly(vinylidene fluoride) hybrid membrane in a flow-through reactor. ACS Appl. Mater. Interfaces 11, 31763–31776 (2019).
Zhao, Y. et al. Janus electrocatalytic flow-through membrane enables highly selective singlet oxygen production. Nat. Commun. 11, 6228 (2020).
Horovitz, I. et al. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: influence of physical parameters. J. Hazard. Mater. 310, 98–107 (2016).
Regmi, C. et al. Comparison of photocatalytic membrane reactor types for the degradation of an organic molecule by TiO2-coated PES membrane. Catalysts 10, 725 (2020).
Fischer, K. et al. Synthesis of high crystalline TiO2 nanoparticles on a polymer membrane to degrade pollutants from water. Catalysts 8, 376 (2018).
Fischer, K. et al. Low-temperature synthesis of anatase/rutile/brookite TiO2 nanoparticles on a polymer membrane for photocatalysis. Catalysts 7, 209 (2017).
Zhang, S., Hedtke, T., Zhou, X., Elimelech, M. & Kim, J.-H. Environmental applications of engineered materials with nanoconfinement. ACS ES T Eng. 1, 706–724 (2021).
Ollis, D. F., Pelizzetti, E. & Serpone, N. Photocatalyzed destruction of water contaminants. Environ. Sci. Technol. 25, 1522–1529 (1991).
Herrmann, J.-M. Photocatalysis fundamentals revisited to avoid several misconceptions. Appl. Catal. B 99, 461–468 (2010).
Renken, A. & Kiwi-Minsker, L. in Advances in Catalysis, Vol. 53 (eds Gates, B. C. & Knözinger, H.) Ch. 2 (Elsevier, 2010).
Gao, Y. et al. Filtration-enhanced highly efficient photocatalytic degradation with a novel electrospun rGO@TiO2 nanofibrous membrane: implication for improving photocatalytic efficiency. Appl. Catal. B 268, 118737 (2020).
Friedmann, D., Mendive, C. & Bahnemann, D. TiO2 for water treatment: parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B 99, 398–406 (2010).
Van der Bruggen, B., Vandecasteele, C., Van Gestel, T., Doyen, W. & Leysen, R. A review of pressure‐driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 22, 46–56 (2003).
Imbrogno, A., Samanta, P. & Schäfer, A. I. Fate of steroid hormone micropollutant estradiol in a hybrid magnetic ion exchange resin-nanofiltration process. Environ. Chem. 16, 630 (2019).
Fogler, H. S. Elements of Chemical Reaction Engineering 5th edn (Pearson, 2016).
Carretero-Genevrier, A., Boissiere, C., Nicole, L. & Grosso, D. Distance dependence of the photocatalytic efficiency of TiO2 revealed by in situ ellipsometry. J. Am. Chem. Soc. 134, 10761–10764 (2012).
Hurwitz, A. R. & Liu, S. T. Determination of aqueous solubility and pKa values of estrogen. J. Pharm. Sci. 66, 624–627 (1977).
Lewis, K. M. & Archer, R. D. pKa values of estrone, 17β-estradiol and 2-methoxyestrone. Steroids 34, 485–499 (1979).
Turchi, C. S. & Ollis, D. F. Photocatalytic degradation of organic water contaminants: mechanisms involving hydroxyl radical attack. J. Catal. 122, 178–192 (1990).
Schäfer, A., Nghiem, L. & Waite, T. Removal of the natural hormone estrone from aqueous solutions using nanofiltration and reverse osmosis. Environ. Sci. Technol. 37, 182–188 (2003).
Ohko, Y. et al. 17β-Estradiol degradation by TiO2 photocatalysis as a means of reducing estrogenic activity. Environ. Sci. Technol. 36, 4175–4181 (2002).
Mai, J., Sun, W., Xiong, L., Liu, Y. & Ni, J. Titanium dioxide mediated photocatalytic degradation of 17β-estradiol in aqueous solution. Chemosphere 73, 600–606 (2008).
Schäfer, A. I., Akanyeti, I. & Semiao, A. J. Micropollutant sorption to membrane polymers: a review of mechanisms for estrogens. Adv. Colloid Interface Sci. 164, 100–117 (2011).
Lyubimenko, R., Richards, B. S., Turshatov, A. & Schäfer, A. I. Separation and degradation detection of nanogram-per-litre concentrations of radiolabelled steroid hormones using combined liquid chromatography and flow scintillation analysis. Sci. Rep. 10, 7095 (2020).
Arlos, M. J. et al. Photocatalytic decomposition of selected estrogens and their estrogenic activity by UV-LED irradiated TiO2 immobilized on porous titanium sheets via thermal-chemical oxidation. J. Hazard. Mater. 318, 541–550 (2016).
Chaves, F. P. et al. Comparative endocrine disrupting compound removal from real wastewater by UV/Cl and UV/H2O2: effect of pH, estrogenic activity, transformation products and toxicity. Sci. Total Environ. 746, 141041 (2020).
Mboula, V. M. et al. Photocatalytic degradation of estradiol under simulated solar light and assessment of estrogenic activity. Appl. Catal. B 162, 437–444 (2015).
Zhang, W., Li, Y., Su, Y., Mao, K. & Wang, Q. Effect of water composition on TiO2 photocatalytic removal of endocrine disrupting compounds (EDCs) and estrogenic activity from secondary effluent. J. Hazard. Mater. 215–216, 252–258 (2012).
Bridle, H. L., Heringa, M. B. & Schäfer, A. I. Solid-phase microextraction to determine micropollutant–macromolecule partition coefficients. Nat. Protoc. 11, 1328–1344 (2016).
Imbrogno, A. & Schäfer, A. I. Comparative study of nanofiltration membrane characterization devices of different dimension and configuration (cross flow and dead end). J. Membr. Sci. 585, 67–80 (2019).
Millipore Express PLUS Membrane GPWP02500 (Millipore, 2017); http://www.merckmillipore.com/DE/en/product/Millipore-Express-PLUS-Membrane-Filter,MM_NF-GPWP02500
Sun, W., Li, S., Mai, J. & Ni, J. Initial photocatalytic degradation intermediates/pathways of 17α-ethynylestradiol: effect of pH and methanol. Chemosphere 81, 92–99 (2010).
O. Levenspiel, Chapter 2. Kinetics of Homogeneous Reactions, in: Chemical Reaction Engineering, Third Edition, John Wiley & Sons, 1998, pp. 13-37.
Lyubimenko, R., Gutierrez Cardenas, O.I., Turshatov, A., Richards, B. S. & Schäfer, A. I. Photodegradation of steroid-hormone micropollutants in a flow-through membrane reactor coated with Pd(II)-porphyrin. Appl. Catal. B 291, 120097 (2021).
Acknowledgements
The Helmholtz Association is thanked for Recruitment Initiative funding, as well as the Helmholtz ERC Recognition Award NAMEPORED to A.I.S. Deutscher Akademischer Austauschdienst (DAAD) provided a PhD stipend to S.L. H. Lambach and S. Schweikert-Joß (IMVT-KIT) are thanked for the fabrication of the photocatalytic membrane cell. At IMT-KIT, B. S. Richards contributed helpful discussions on light data analysis and T. Berger provided support in micro-cross-flow system troubleshooting, LabVIEW programming and light absorption measurements. At Leibniz-IOM, A. Prager supplied SEM images and A. A. Latif performed the TiO2 coating of membranes. T. Luxbacher (Anton Paar) and J. Lützenkirchen (INE-KIT) contributed expertise with streaming potential measurements. At IAMT, R. Lyubimenko developed the UHPLC analysis, assisted with operation and contributed to discussion of calculation methods, C. Regmi prepared membrane cross-sections, J. C. Espíndola discussed aspects of data interpretation, A. Imbrogno established the error calculation methodology, M. Nguyen supported with LabVIEW operation, IT troubleshooting, provided hormone properties and took care of regraphing and error corrections, E. Véron carried out E2 photocatalytic degradation experiments with the TiO2_120C membrane, and C. Suliani Raota carried out repeat experiments with methylene blue. Last but not least, we express our sincere gratitude to F.-D. Kopinke (UFZ Leipzig) for his post-acceptance review that helped identify errors and significantly improve the manuscript.
Author information
Authors and Affiliations
Contributions
A.I.S. developed the concept and methodology of the project. S.L. performed photocatalytic experiments and analysed the data. K.F. and A.S. developed and synthesized the photocatalysts. S.L. wrote the original manuscript, and A.I.S., K.F. and A.S. edited and reviewed the manuscript. K.F. and A.S. provided the resources for nanoparticle synthesis and membrane coating. A.I.S. provided the funding and resources for photocatalytic membrane filtration and sample analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supporting information, Figs. 1–12 and Tables 1–4.
Rights and permissions
About this article
Cite this article
Lotfi, S., Fischer, K., Schulze, A. et al. Photocatalytic degradation of steroid hormone micropollutants by TiO2-coated polyethersulfone membranes in a continuous flow-through process. Nat. Nanotechnol. 17, 417–423 (2022). https://doi.org/10.1038/s41565-022-01074-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01074-8
This article is cited by
-
An enzymatic continuous-flow reactor based on a pore-size matching nano- and isoporous block copolymer membrane
Nature Communications (2024)
-
A critical and comprehensive review of the current status of 17β-estradiol hormone remediation through adsorption technology
Environmental Science and Pollution Research (2024)
-
Customized carbon composite nanomaterials for the mitigation of emerging contaminants: a review of recent trends
Carbon Letters (2024)
-
Design of resilient and viable sourcing strategies in intertwined circular supply networks
Annals of Operations Research (2024)
-
Environmental impacts and remediation of dye-containing wastewater
Nature Reviews Earth & Environment (2023)