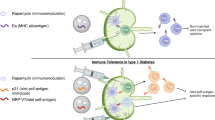
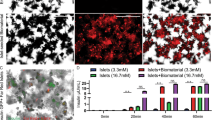
Abstract
Standard oral rapamycin (that is, Rapamune) administration is plagued by poor bioavailability and broad biodistribution. Thus, this pleotropic mammalian target of rapamycin (mTOR) inhibitor has a narrow therapeutic window and numerous side effects and provides inadequate protection to transplanted cells and tissues. Furthermore, the hydrophobicity of rapamycin limits its use in parenteral formulations. Here, we demonstrate that subcutaneous delivery via poly(ethylene glycol)-b-poly(propylene sulfide) polymersome nanocarriers significantly alters rapamycin’s cellular biodistribution to repurpose its mechanism of action for tolerance, instead of immunosuppression, and minimize side effects. While oral rapamycin inhibits T cell proliferation directly, subcutaneously administered rapamycin-loaded polymersomes modulate antigen presenting cells in lieu of T cells, significantly improving maintenance of normoglycemia in a clinically relevant, major histocompatibility complex-mismatched, allogeneic, intraportal (liver) islet transplantation model. These results demonstrate the ability of a rationally designed nanocarrier to re-engineer the immunosuppressive mechanism of a drug by controlling cellular biodistribution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Raw RNA-seq reads and data are accessible through GEO Series accession number GSE182776. Other raw and analysed datasets generated during the study are available for research purposes from the corresponding author on reasonable request.
References
Shapiro, A. M., Pokrywczynska, M. & Ricordi, C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 13, 268–277 (2017).
Molano, R. D. et al. Long-term islet allograft survival in nonobese diabetic mice treated with tacrolimus, rapamycin, and anti-interleukin-2 antibody. Transplantation 75, 1812–1819 (2003).
Shapiro, A. M. et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238 (2000).
Rapamune (sirolimus) [Package Insert]. Wyeth Pharmaceuticals, Collegeville, PA (2011).
Halloran, P. F. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 351, 2715–2729 (2004).
Yatscoff, R. W., Wang, P., Chan, K., Hicks, D. & Zimmerman, J. Rapamycin: distribution, pharmacokinetics, and therapeutic range investigations. Ther. Drug Monit. 17, 666–671 (1995).
Ferron, G. M., Mishina, E. V., Zimmerman, J. J. & Jusko, W. J. Population pharmacokinetics of sirolimus in kidney transplant patients. Clin. Pharmacol. Ther. 61, 416–428 (1997).
Meier-Kriesche, H. U. & Kaplan, B. Toxicity and efficacy of sirolimus: relationship to whole-blood concentrations. Clin. Ther. 22, B93–B100 (2000).
Hafiz, M. M. et al. Immunosuppression and procedure-related complications in 26 patients with type 1 diabetes mellitus receiving allogeneic islet cell transplantation. Transplantation 80, 1718–1728 (2005).
Lombardi, G. & Vasquez, Y. in Handbook of Experimental Pharmacology, Vol. 188 (eds Lombardi, G. & Vasquez, Y.) Preface (Springer, 2009).
Stabler, C. L., Li, Y., Stewart, J. M. & Keselowsky, B. C. Engineering immunomodulatory biomaterials for type 1 diabetes. Nat. Rev. Mater. 4, 429–450 (2019).
Emoto, C., Fukuda, T., Cox, S., Christians, U. & Vinks, A. A. Development of a physiologically-based pharmacokinetic model for sirolimus: predicting bioavailability based on intestinal CYP3A content. CPT Pharmacometrics Syst. Pharmacol. 2, e59 (2013).
Haeri, A., Osouli, M., Bayat, F., Alavi, S. & Dadashzadeh, S. Nanomedicine approaches for sirolimus delivery: a review of pharmaceutical properties and preclinical studies. Artif. Cells Nanomed. Biotechnol. 46, 1–14 (2018).
Alemdar, A. Y., Baker, K. A., Sadi, D., McAlister, V. C. & Mendez, I. Liposomal tacrolimus administered systemically and within the donor cell suspension improves xenograft survival in hemiparkinsonian rats. Exp. Neurol. 172, 416–424 (2001).
Haeri, A. et al. Use of remote film loading methodology to entrap sirolimus into liposomes: preparation, characterization and in vivo efficacy for treatment of restenosis. Int. J. Pharm. 414, 16–27 (2011).
Allen, S., Osorio, O., Liu, Y. G. & Scott, E. Facile assembly and loading of theranostic polymersomes via multi-impingement flash nanoprecipitation. J. Control. Release 262, 91–103 (2017).
Allen, S. D. et al. Polymersomes scalably fabricated via flash nanoprecipitation are non-toxic in non-human primates and associate with leukocytes in the spleen and kidney following intravenous administration. Nano Res. https://doi.org/10.1007/s12274-018-2069-x (2018).
Stano, A., Scott, E. A., Dane, K. Y., Swartz, M. A. & Hubbell, J. A. Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials 34, 4339–4346 (2013).
Scott, E. A. et al. Dendritic cell activation and T cell priming with adjuvant- and antigen-loaded oxidation-sensitive polymersomes. Biomaterials 33, 6211–6219 (2012).
Dowling, D. J. et al. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 140, 1339–1350 (2017).
Yi, S. et al. Tailoring nanostructure morphology for enhanced targeting of dendritic cells in atherosclerosis. ACS Nano 10, 11290–11303 (2016).
Bracho-Sanchez, E., Hassanzadeh, A., Brusko, M. A., Wallet, M. A. & Keselowsky, B. G. Dendritic cells treated with exogenous indoleamine 2,3-dioxygenase maintain an immature phenotype and suppress antigen-specific T cell proliferation. J. Immunol. Regen. Med. https://doi.org/10.1016/j.regen.2019.100015 (2019).
Peng, Y., Latchman, Y. & Elkon, K. B. Ly6Clow monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. J. Immunol. 182, 2777–2785 (2009).
Allen, R. P., Bolandparvaz, A., Ma, J. A., Manickam, V. A. & Lewis, J. S. Latent, immunosuppressive nature of poly(lactic-co-glycolic acid) microparticles. ACS Biomater. Sci. Eng. 4, 900–918 (2018).
Zhang, N. et al. Sirolimus is associated with reduced islet engraftment and impaired β-cell function. Diabetes 55, 2429–2436 (2006).
Rosborough, B. R. et al. Adenosine triphosphate-competitive mTOR inhibitors: a new class of immunosuppressive agents that inhibit allograft rejection. Am. J. Transpl. 14, 2173–2180 (2014).
van den Bosch, T. P., Kannegieter, N. M., Hesselink, D. A., Baan, C. C. & Rowshani, A. T. Targeting the monocyte-macrophage lineage in solid organ transplantation. Front. Immunol. 8, 153 (2017).
Abbas, A. K. & Lichtman, A. H. Basic Immunology: Functions and Disorders of the Immune System 2nd edn (Saunders, 2004).
Cantarelli, E. et al. Murine animal models for preclinical islet transplantation: no model fits all (research purposes). Islets 5, 79–86 (2013).
Mahe, E. et al. Cutaneous adverse events in renal transplant recipients receiving sirolimus-based therapy. Transplantation 79, 476–482 (2005).
Ventola, C. L. Progress in nanomedicine: approved and investigational nanodrugs. P T 42, 742–755 (2017).
Burrack, A. L., Martinov, T. & Fife, B. T. T cell-mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Front. Endocrinol. (Lausanne) 8, 343 (2017).
Bouhdoud, L., Villain, P., Merzouki, A., Arella, M. & Couture, C. T-cell receptor-mediated anergy of a human immunodeficiency virus (HIV) gp120-specific CD4+ cytotoxic T-cell clone, induced by a natural HIV type 1 variant peptide. J. Virol. 74, 2121–2130 (2000).
Vieyra-Lobato, M. R., Vela-Ojeda, J., Montiel-Cervantes, L., Lopez-Santiago, R. & Moreno-Lafont, M. C. Description of CD8+ regulatory T lymphocytes and their specific intervention in graft-versus-host and infectious diseases, autoimmunity, and cancer. J. Immunol. Res. 2018, 3758713 (2018).
Fu, C. et al. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc. Natl Acad. Sci. USA 117, 23730–23741 (2020).
Thomas, H. E., Darwiche, R., Corbett, J. A. & Kay, T. W. Interleukin-1 plus γ-interferon-induced pancreatic β-cell dysfunction is mediated by β-cell nitric oxide production. Diabetes 51, 311–316 (2002).
Kawamura, S. & Ohteki, T. Monopoiesis in humans and mice. Int. Immunol. 30, 503–509 (2018).
Zhu, J., Chen, H., Huang, X., Jiang, S. & Yang, Y. Ly6Chi monocytes regulate T cell responses in viral hepatitis. JCI Insight 1, e89880 (2016).
Parrot, T. et al. Transcriptomic features of tumour-infiltrating CD4lowCD8high double positive ɑβ T cells in melanoma. Sci. Rep. 10, 5900 (2020).
Parel, Y. et al. Presence of CD4+CD8+ double-positive T cells with very high interleukin-4 production potential in lesional skin of patients with systemic sclerosis. Arthritis Rheum. 56, 3459–3467 (2007).
Overgaard, N. H., Jung, J. W., Steptoe, R. J. & Wells, J. W. CD4+/CD8+ double-positive T cells: more than just a developmental stage? J. Leukoc. Biol. 97, 31–38 (2015).
Dew, M. A. et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation 83, 858–873 (2007).
Nulojix (belatacept) [Package Insert]. Bristol Myers Squibb, Princeton, NJ (2011).
O’Hare, F. M. et al. Neutrophil and monocyte toll-like receptor 4, CD11b and reactive oxygen intermediates, and neuroimaging outcomes in preterm infants. Pediatr. Res. 78, 82–90 (2015).
Yasunami, Y. et al. Vɑ14 NK T cell-triggered IFN-γ production by Gr-1+CD11b+ cells mediates early graft loss of syngeneic transplanted islets. J. Exp. Med. 202, 913–918 (2005).
Manzoli, V. et al. Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol. Am. J. Transpl. 18, 590–603 (2018).
Allen, S. D., Bobbala, S., Karabin, N. B., Modak, M. & Scott, E. A. Benchmarking bicontinuous nanospheres against polymersomes for in vivo biodistribution and dual intracellular delivery of lipophilic and water-soluble payloads. ACS Appl. Mater. Interfaces 10, 33857–33866 (2018).
Yu, Y. R. et al. A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PLoS ONE 11, e0150606 (2016).
Belkina, A. C. et al. Automated optimized parameters for T-distributed stochastic neighbor embedding improve visualization and analysis of large datasets. Nat. Commun. 10, 5415 (2019).
Andrews, S. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc (Babraham Bioinformatics, 2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Trapnell, C. et al. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Trapnell, C. et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31, 46–53 (2013).
Roberts, A., Trapnell, C., Donaghey, J., Rinn, J. L. & Pachter, L. Improving RNA-seq expression estimates by correcting for fragment bias. Genome Biol. 12, R22 (2011).
Acknowledgements
A.D. Jerez designed and created the illustration in Fig. 1. Modifications were made by J.A.B. This research is based on work supported by the National Science Foundation Graduate Research Fellowship under grant no. DGE-1842165. This work was funded in part by the National Institutes of Health (NIH grant no. 1DP2HL132390-01); the National Science Foundation (NSF grant no. DGE-1842165); the Center for Advanced Regenerative Engineering (CARE) at Northwestern University; services and equipment were used at the Flow Cytometry Facility at the University of Chicago; the Integrated Molecular Structure Education and Research Center (IMSERC) at Northwestern University, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF grant no. ECCS-1542205), the State of Illinois and the International Institute for Nanotechnology (IIN); the Northwestern University Center for Advanced Molecular Imaging (CAMI), which is supported by NCI grant no. CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center; the BioCryo facility of Northwestern University’s NUANCE Center, which has received support from the SHyNE Resource (NSF grant no. ECCS-1542205); the MRSEC program (NSF grant no. DMR-1720139) at the Materials Research Center; the IIN; and the State of Illinois, through the IIN; and Northwestern University NUSeq Core Facility (NSF grant no. DMR-1229693). SAXS experiments were performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the Advanced Photon Source (APS). DND-CAT is supported by Northwestern University, The Dow Chemical Company, and DuPont de Nemours, Inc. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Data was collected using an instrument funded by the National Science Foundation under Award No. 0960140.
Author information
Authors and Affiliations
Contributions
J.A.B. designed the experiments with the assistance of S.D.A. J.A.B., X.Z., S.B., M.A.F., C.B.F. and H.F.H. performed the experiments. R.A.K.R. performed computational analysis on the RNA-sequencing data. J.A.B. analysed the data and composed the manuscript. E.A.S. and G.A.A. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
J.A.B., S.D.A., E.A.S. and G.A.A. are coinventors on a patient application related to the work presented in this manuscript. The other authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10 and Tables 1–28.
Rights and permissions
About this article
Cite this article
Burke, J.A., Zhang, X., Bobbala, S. et al. Subcutaneous nanotherapy repurposes the immunosuppressive mechanism of rapamycin to enhance allogeneic islet graft viability. Nat. Nanotechnol. 17, 319–330 (2022). https://doi.org/10.1038/s41565-021-01048-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-01048-2
This article is cited by
-
Challenges and future directions for next-generation biomedical polymersomes
Science China Materials (2024)
-
Therapeutic induction of antigen-specific immune tolerance
Nature Reviews Immunology (2023)
-
Engineering nanomaterial physical characteristics for cancer immunotherapy
Nature Reviews Bioengineering (2023)
-
Targeted modulation of immune cells and tissues using engineered biomaterials
Nature Reviews Bioengineering (2023)
-
Screening of Diabetic Nephropathy Progression-Related Genes Based on Weighted Gene Co-expression Network Analysis
Biochemical Genetics (2023)