Abstract
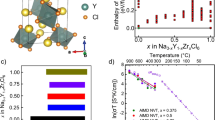
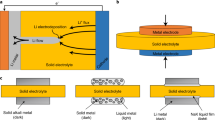
Solid-state sodium (Na) batteries have received extensive attention as a promising alternative to room-temperature liquid electrolyte Na-ion batteries and high-temperature liquid electrode Na–S batteries because of safety concerns. However, the major issues for solid-state Na batteries are a high interfacial resistance between solid electrolytes and electrodes, and Na dendrite growth. Here we report that a yttria-stabilized zirconia (YSZ)-enhanced beta-alumina solid electrolyte (YSZ@BASE) has an extremely low interface impedance of 3.6 Ω cm2 with the Na metal anode at 80 °C, and also exhibits an extremely high critical current density of ~7.0 mA cm–2 compared with those of other Li- and Na-ion solid electrolytes reported so far. With a trace amount of eutectic NaFSI–KFSI molten salt at the electrolyte/cathode interface, a quasi-solid-state Na/YSZ@BASE/NaNi0.45Cu0.05Mn0.4Ti0.1O2 full cell achieves a high capacity of 110 mAh g–1 with a Coulombic efficiency >99.99% and retains 73% of the cell capacity over 500 cycles at 4C and 80 °C. Extensive characterizations and theoretical calculations prove that the stable β-NaAlO2-rich solid–electrolyte interphase and strong YSZ support matrix play a critical role in suppressing the Na dendrite as they maintain robust interfacial contacts, lower electronic conduction and prevent the continual reduction of BASE through oxygen-ion compensation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data that support the plots within this article and other findings of this study are available from the corresponding authors upon request.
References
Tarascon, J. M. Is lithium the new gold? Nat. Chem. 2, 510 (2010).
Slater, M. D., Kim, D., Lee, E. & Johnson, C. S. Sodium-ion batteries. Adv. Funct. Mater. 23, 947–958 (2013).
Fan, X. et al. High-performance all-solid-state Na–S battery enabled by casting–annealing technology. ACS Nano 12, 3360–3368 (2018).
Zhou, W., Li, Y., Xin, S. & Goodenough, J. B. Rechargeable sodium all-solid-state battery. ACS Cent. Sci. 3, 52–57 (2017).
Zhao, C. et al. Solid-state sodium batteries. Adv. Energy Mater. 8, 1703012 (2018).
Goodenough, J. B., Hong, H.-P. & Kafalas, J. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 11, 203–220 (1976).
Lu, Y., Li, L., Zhang, Q., Niu, Z. & Chen, J. Electrolyte and interface engineering for solid-state sodium batteries. Joule 2, 1747–1770 (2018).
Hou, W. et al. Solid electrolytes and interfaces in all-solid-state sodium batteries: progress and perspective. Nano Energy 52, 279–291 (2018).
Hayashi, A., Noi, K., Sakuda, A. & Tatsumisago, M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 3, 856 (2012).
Berbano, S. S., Seo, I., Bischoff, C. M., Schuller, K. E. & Martin, S. W. Formation and structure of Na2S + P2S5 amorphous materials prepared by melt-quenching and mechanical milling. J. Non Cryst. Solids 358, 93–98 (2012).
Heo, J. W., Banerjee, A., Park, K. H., Jung, Y. S. & Hong, S.-T. New Na-ion solid electrolytes Na4–xSn1−xSbxS4 (0.02 ≤ x ≤ 0.33) for all-solid-state Na-ion batteries. Adv. Energy Mater. 8, 1702716 (2018).
Lu, X., Xia, G., Lemmon, J. P. & Yang, Z. Advanced materials for sodium–beta alumina batteries: status, challenges and perspectives. J. Power Sources 195, 2431–2442 (2010).
Lu, X. et al. Liquid–metal electrode to enable ultra-low temperature sodium–beta alumina batteries for renewable energy storage. Nat. Commun. 5, 4578 (2014).
Näfe, H., Fritz, M. & Lorenz, W. On the defect electron conduction parameter of Na·beta-alumina. Solid State Ion 74, 275–278 (1994).
Lu, X. et al. Advanced intermediate-temperature Na–S battery. Energy Environ. Sci. 6, 299–306 (2013).
Li, G. et al. Advanced intermediate temperature sodium–nickel chloride batteries with ultra-high energy density. Nat. Commun. 7, 10683 (2016).
Wen, Z. et al. Research on sodium sulfur battery for energy storage. Solid State Ion 179, 1697–1701 (2008).
Wen, Z. et al. Research activities in Shanghai Institute of Ceramics, Chinese Academy of Sciences on the solid electrolytes for sodium sulfur batteries. J. Power Sources 184, 641–645 (2008).
De Jonghe, L. C., Feldman, L. & Beuchele, A. Slow degradation and electron conduction in sodium/beta-aluminas. J. Mater. Sci. 16, 780–786 (1981).
Williams, R. M. et al. The thermal stability of sodium beta''-alumina solid electrolyte ceramic in AMTEC cells. In Space Technology and Applications International Forum 1306–1311 (AIP, 1999).
Ansell, R. The chemical and electrochemical stability of beta-alumina. J. Mater. Sci. 21, 365–379 (1986).
Bay, M. C. et al. Sodium plating from Na‐β″‐alumina ceramics at room temperature, paving the way for fast‐charging all‐solid‐state batteries. Adv. Energy Mater. 10, 1902899 (2020).
Lacivita, V., Wang, Y., Bo, S.-H. & Ceder, G. Ab initio investigation of the stability of electrolyte/electrode interfaces in all-solid-state Na batteries. J. Mater. Chem. A 7, 8144–8155 (2019).
Krauskopf, T., Mogwitz, B., Rosenbach, C., Zeier, W. G. & Janek, J. Diffusion limitation of lithium metal and Li–Mg alloy anodes on LLZO type solid electrolytes as a function of temperature and pressure. Adv. Energy Mater. 9, 1902568 (2019).
Kasemchainan, J. et al. Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nat. Mater. 18, 1105–1111 (2019).
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Bianchini, F., Fjellvag, H. & Vajeeston, P. A first principle comparative study of the ionic diffusivity in LiAlO2 and NaAlO2 polymorphs for solid-state battery applications. Phys. Chem. Chem. Phys. 20, 9824–9832 (2018).
Krauskopf, T., Richter, F. H., Zeier, W. G. & Janek, J. R. Physicochemical concepts of the lithium metal anode in solid-state batteries. Chem. Rev. 120, 7745–7794 (2020).
Aetukuri, N. B. et al. Flexible ion‐conducting composite membranes for lithium batteries. Adv. Energy Mater. 5, 1500265 (2015).
Cho, Y.-H. et al. Mechanical properties of the solid Li-ion conducting electrolyte: Li0.33La0.57TiO3. J. Mater. Sci. 47, 5970–5977 (2012).
Krauskopf, T., Hartmann, H., Zeier, W. G. & Janek, J. Toward a fundamental understanding of the lithium metal anode in solid-state batteries-an electrochemo-mechanical study on the garnet-type solid electrolyte Li6.25Al0.25La3Zr2O12. ACS Appl. Mater. Interfaces 11, 14463–14477 (2019).
Kim, Y. et al. The effect of relative density on the mechanical properties of hot‐pressed cubic Li7La3Zr2O12. J. Am. Ceram. Soc. 99, 1367–1374 (2016).
Wolfenstine, J., Allen, J. L., Sakamoto, J., Siegel, D. J. & Choe, H. Mechanical behavior of Li-ion-conducting crystalline oxide-based solid electrolytes: a brief review. Ionics 24, 1271–1276 (2017).
De Jonghe, L. C. Transport number gradients and solid electrolyte degradation. J. Electrochem. Soc. 129, 752–755 (1982).
Zhang, L., Zhu, L. & Virkar, A. V. Electronic conductivity measurement of yttria-stabilized zirconia solid electrolytes by a transient technique. J. Power Sources 302, 98–106 (2016).
Rangasamy, E., Wolfenstine, J. & Sakamoto, J. The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ion 206, 28–32 (2012).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Shin, B. R. et al. Comparative study of TiS2/Li–In all-solid-state lithium batteries using glass-ceramic Li3PS4 and Li10GeP2S12 solid electrolytes. Electrochim. Acta 146, 395–402 (2014).
Wagner, C. Adsorbed atomic species as intermediates in heterogeneous catalysis. Adv. Catal. 21, 323–381 (1970).
De Jonghe, L. C. & Buechele, A. Chemical colouration of sodium beta-aluminas. J. Mater. Sci. 17, 885–892 (1982).
De Jonghe, L. C., Buechele, A. & Armand, M. Oxygen interstitial transport and chemical coloration in sodium-beta alumina. Solid State Ion. 9, 165–168 (1983).
Weber, N. A thermoelectric device based on beta-alumina solid electrolyte. Energy Convers. 14, 1–8 (1974).
Westover, A. S., Dudney, N. J., Sacci, R. L. & Kalnaus, S. Deposition and confinement of Li metal along an artificial Lipon–Lipon interface. ACS Energy Lett. 4, 651–655 (2019).
Porz, L. et al. Mechanism of lithium metal penetration through inorganic solid electrolytes. Adv. Energy Mater. 7, 1701003 (2017).
Yao, H. R. et al. Designing air-stable O3-type cathode materials by combined structure modulation for Na-ion batteries. J. Am. Chem. Soc. 139, 8440–8443 (2017).
Fukunaga, A. et al. Intermediate-temperature ionic liquid NaFSA–KFSA and its application to sodium secondary batteries. J. Power Sources 209, 52–56 (2012).
Kubota, K., Nohira, T., Goto, T. & Hagiwara, R. Novel inorganic ionic liquids possessing low melting temperatures and wide electrochemical windows: binary mixtures of alkali bis(fluorosulfonyl)amides. Electrochem. Commun. 10, 1886–1888 (2008).
Bish, D. L. & Howard, S. Quantitative phase analysis using the Rietveld method. J. Appl. Crystallogr. 21, 86–91 (1988).
Cheary, R. W. & Coelho, A. A fundamental parameters approach to X-ray line-profile fitting. J. Appl. Crystallogr. 25, 109–121 (1992).
Reed, D. et al. Wetting of sodium on β″-Al2O3/YSZ composites for low temperature planar sodium-metal halide batteries. J. Power Sources 227, 94–100 (2013).
Huntington, H. B. Ultrasonic measurements on single crystals. Phys. Rev. 72, 321 (1947).
de With, G. & Wagemans, H. H. Ball-on-ring test revisited. J. Am. Ceram. Soc. 72, 1538–1541 (1989).
Han, F., Zhu, Y., He, X., Mo, Y. & Wang, C. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv. Energy Mater. 6, 1501590 (2016).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996).
Ong, S. P., Wang, L., Kang, B. & Ceder, G. Li−Fe−P−O2 phase diagram from first principles calculations. Chem. Mater. 20, 1798–1807 (2008).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Young, T.III. An essay on the cohesion of fluid. Phil. Trans. R. Soc. Lond. 1805, 65–87 (1805).
Kim, Y., Ko, W.-S. & Lee, B.-J. Second nearest-neighbor modified embedded atom method interatomic potentials for the Na unary and Na–Sn binary systems. Comp. Mater. Sci. 185, 109953 (2020).
Acknowledgements
This work was supported by US Department of Energy (DOE) under award no. DEEE0008856 at the University of Maryland (UMD) and Office of Electricity (OE) at Pacific Northwest National Laboratory (PNNL). We appreciate useful discussions with I. Gyuk of the DOE-OE Grid Storage Program. T.D. is grateful for financial support from the Engie Chuck Edwards Memorial Fellowship at UMD. Additional support is from North Carolina A&T State University (NC A&T) through a faculty start-up fund. Some of the TEM and XPS analyses were conducted in the William R. Wiley Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the DOE’s Office of Biological and Environmental Research and located at PNNL. PNNL is a multiprogram laboratory operated by Battelle Memorial Institute for the DOE under contract DE-AC05-76RL01830. We also thank J. F. Bonnett for the YSZ@BASE conversion, J. Song for cathode preparation, M. E. Bowden for the XRD analysis, and M. H. Engelhard for the XPS characterization. Part of the SEM analysis was done by K. Nowlin at the Joint School of Nanoscience and Nanoengineering, an academic collaboration between NC A&T and the University of North Carolina at Greensboro.
Author information
Authors and Affiliations
Contributions
T.D., Chunsheng Wang and X.L. proposed the research and designed the experiment. T.D., X.L., O.C., L.C., T.R.A. and H.-J.C. performed the experiments (material synthesis, characterization, battery fabrication and testing, and so on). X.J., T.D., Chungsheng Wang and X.L. conceived the simulation protocol, and X.J. carried out the simulations. L.Z., Chongmin Wang and X.F. performed the TEM, FIB, STEM–EDS analyses. T.D., Chungsheng Wang and X.L. analysed the data and wrote the manuscript with suggestions and comments from all the authors. All the authors contributed to the interpretation of the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Yoon Seok Jung, Guoxiu Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–30, Discussion and Tables 1–4.
Rights and permissions
About this article
Cite this article
Deng, T., Ji, X., Zou, L. et al. Interfacial-engineering-enabled practical low-temperature sodium metal battery. Nat. Nanotechnol. 17, 269–277 (2022). https://doi.org/10.1038/s41565-021-01036-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-01036-6
This article is cited by
-
Developing trends in nanomaterials and their environmental implications
Nature Nanotechnology (2023)
-
Suppressing Dendrite Growth with Quasi-liquid Anode in Solid-State Sodium Metal Batteries Enabled by the Design of Na-K Alloying Strategy
Journal of Electronic Materials (2023)