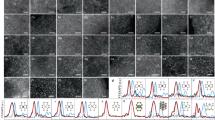
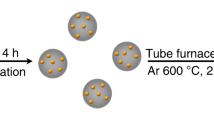
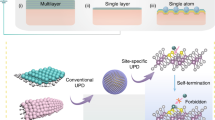
Abstract
The stabilization of transition metals as isolated centres with high areal density on suitably tailored carriers is crucial for maximizing the industrial potential of single-atom heterogeneous catalysts. However, achieving single-atom dispersions at metal contents above 2 wt% remains challenging. Here we introduce a versatile approach combining impregnation and two-step annealing to synthesize ultra-high-density single-atom catalysts with metal contents up to 23 wt% for 15 metals on chemically distinct carriers. Translation to a standardized, automated protocol demonstrates the robustness of our method and provides a path to explore virtually unlimited libraries of mono- or multimetallic catalysts. At the molecular level, characterization of the synthesis mechanism through experiments and simulations shows that controlling the bonding of metal precursors with the carrier via stepwise ligand removal prevents their thermally induced aggregation into nanoparticles. The drastically enhanced reactivity with increasing metal content exemplifies the need to optimize the surface metal density for a given application. Moreover, the loading-dependent site-specific activity observed in three distinct catalytic systems reflects the well-known complexity in heterogeneous catalyst design, which now can be tackled with a library of single-atom catalysts with widely tunable metal loadings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the findings of this study have been included in the main text and Supplementary Information. Any additional materials and data are available from the corresponding author upon reasonable request.
Change history
04 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41565-022-01090-8
References
Kaiser, S. K., Chen, Z., Faust Akl, D., Mitchell, S. & Pérez-Ramírez, J. Single-atom catalysts across the periodic table. Chem. Rev. 120, 11703–11809 (2020).
Li, Z. et al. Well-defined materials for heterogeneous catalysis: from nanoparticles to isolated single-atom sites. Chem. Rev. 120, 623–682 (2019).
Li, X., Yang, X., Huang, Y., Zhang, T. & Liu, B. Supported noble‐metal single atoms for heterogeneous catalysis. Adv. Mater. 31, 1902031 (2019).
Wang, Y. et al. Catalysis with two-dimensional materials confining single atoms: concept, design, and applications. Chem. Rev. 119, 1806–1854 (2018).
Lin, L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80–83 (2017).
Datye, A. K. & Guo, H. Single atom catalysis poised to transition from an academic curiosity to an industrially relevant technology. Nat. Commun. 12, 895 (2021).
Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Zhang, L., Zhou, M., Wang, A. & Zhang, T. Selective hydrogenation over supported metal catalysts: from nanoparticles to single atoms. Chem. Rev. 120, 683–733 (2019).
Liu, D. et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 4, 512–518 (2019).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Chen, Z. et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling. Nat. Nanotechnol. 13, 702–707 (2018).
Jung, E. et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020).
Beniya, A. & Higashi, S. Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2, 590–602 (2019).
Ji, S. et al. Chemical synthesis of single atomic site catalysts. Chem. Rev. 120, 11900–11955 (2020).
Ding, S., Hülsey, M. J., Pérez-Ramírez, J. & Yan, N. Transforming energy with single-atom catalysts. Joule 3, 2897–2929 (2019).
DeRita, L. et al. Catalyst architecture for stable single atom dispersion enables site-specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters, and metallic Pt clusters on TiO2. J. Am. Chem. Soc. 139, 14150–14165 (2017).
Yang, H. B. et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Liu, Y. et al. A general strategy for fabricating isolated single metal atomic site catalysts in Y zeolite. J. Am. Chem. Soc. 141, 9305–9311 (2019).
Fei, H. et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018).
He, X. et al. A versatile route to fabricate single atom catalysts with high chemoselectivity and regioselectivity in hydrogenation. Nat. Commun. 10, 3663 (2019).
Wei, H. et al. Iced photochemical reduction to synthesize atomically dispersed metals by suppressing nanocrystal growth. Nat. Commun. 8, 1490 (2017).
Yang, H. et al. A universal ligand mediated method for large scale synthesis of transition metal single atom catalysts. Nat. Commun. 10, 4585 (2019).
Zhang, Z. et al. Electrochemical deposition as a universal route for fabricating single-atom catalysts. Nat. Commun. 11, 1215 (2020).
Zhao, L. et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 10, 1278 (2019).
Wei, S. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 13, 856–861 (2018).
Yao, Y. et al. High temperature shockwave stabilized single atoms. Nat. Nanotechnol. 14, 851–857 (2019).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Wang, L. et al. A sulfur-tethering synthesis strategy toward high-loading atomically dispersed noble metal catalysts. Sci. Adv. 5, eaax6322 (2019).
Cheng, Y. et al. Atomically dispersed transition metals on carbon nanotubes with ultrahigh loading for selective electrochemical carbon dioxide reduction. Adv. Mater. 30, 1706287 (2018).
Kunwar, D. et al. Stabilizing high metal loadings of thermally stable platinum single atoms on an industrial catalyst support. ACS Catal. 9, 3978–3990 (2019).
Zhang, L. et al. Direct observation of dynamic bond evolution in single‐atom Pt/C3N4 catalysts. Angew. Chem. Int. Ed. 59, 6224–6229 (2020).
Li, H. et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 13, 411–417 (2018).
Avakyan, L. et al. Atomic structure of nickel phthalocyanine probed by X-ray absorption spectroscopy and density functional simulations. Opt. Spectrosc. 114, 347–352 (2013).
Kabir, S., Artyushkova, K., Serov, A., Kiefer, B. & Atanassov, P. Binding energy shifts for nitrogen‐containing graphene‐based electrocatalysts-experiments and DFT calculations. Surf. Interface Anal. 48, 293–300 (2016).
Jiang, K. et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 11, 893–903 (2018).
Kim, H. et al. Identification of single-atom Ni site active toward electrochemical CO2 conversion to CO. J. Am. Chem. Soc. 143, 925–933 (2021).
Kaiser, S. K. et al. Nanostructuring unlocks high performance of platinum single-atom catalysts for stable vinyl chloride production. Nat. Catal. 3, 376–385 (2020).
Du, Y. et al. XAFCA: a new XAFS beamline for catalysis research. J. Synchrotron Radiat. 22, 839–843 (2015).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Henkelman, G. & Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 111, 7010–7022 (1999).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
J. Lu acknowledges support from MOE grant (R-143-000-B47-114), the Ministry of Education (Singapore) through the Research Centre of Excellence program (Award EDUN C-33-18-279-V12, Institute for Functional Intelligent Materials) and the National University of Singapore Flagship Green Energy Program (R-143-000-A55-646). X.Z. acknowledges support from a Presidential Postdoctoral Fellowship, Nanyang Technological University, Singapore via grant 03INS000973C150. S.M., D.F.A., and J.P.-R. acknowledge funding from the NCCR Catalysis, a National Centre of Competence in Research funded by the Swiss National Science Foundation. Jun Li acknowledges financial support by the National Natural Science Foundation of China (grant number 22033005) and the Guangdong Provincial Key Laboratory of Catalysis (2020B121201002). Computational resources were supported by the Center for Computational Science and Engineering (SUSTech) and Tsinghua National Laboratory for Information Science and Technology. We would like to acknowledge the Facility for Analysis, Characterization, Testing and Simulation, Nanyang Technological University, Singapore, for use of their electron microscopy facilities.
Author information
Authors and Affiliations
Contributions
X.H. and J. Lu conceived and designed the experiments. J.P.-R. conceived the automated synthesis protocol. J. Lu and J.P.-R. supervised the project and organized the collaboration. X.H. performed materials synthesis. S.M. and D.F.A performed automated synthesis. X.H. and T.S. performed the activity test. S.X. performed the XAFS measurement. X.Z., H.X. and D.K. performed the electron microscopy experiments and data analysis. C.S. helped to perform the CO-DRIFTS measurements. Jing Li performed the XPS measurements. Z.L. performed the nitrogen sorption measurements. H.Y. and Y.C. performed the X-ray diffraction measurements. K.H. and Jun Li carried out theoretical calculations. X.H., S.M., J.P.-R., Jun Li and J. Lu co-wrote the manuscript. All authors discussed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Abhaya Datye and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31 and Tables 1–3.
Rights and permissions
About this article
Cite this article
Hai, X., Xi, S., Mitchell, S. et al. Scalable two-step annealing method for preparing ultra-high-density single-atom catalyst libraries. Nat. Nanotechnol. 17, 174–181 (2022). https://doi.org/10.1038/s41565-021-01022-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-01022-y
This article is cited by
-
Transcending scales in catalysis for sustainable development
Nature Chemical Engineering (2024)
-
Sequential co-reduction of nitrate and carbon dioxide enables selective urea electrosynthesis
Nature Communications (2024)
-
Tailoring d-band center of high-valent metal-oxo species for pollutant removal via complete polymerization
Nature Communications (2024)
-
In situ modulating coordination fields of single-atom cobalt catalyst for enhanced oxygen reduction reaction
Nature Communications (2024)
-
Continuous and low-carbon production of biomass flash graphene
Nature Communications (2024)