Abstract
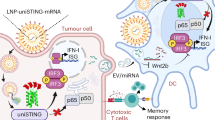
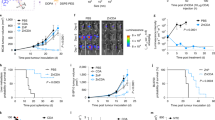
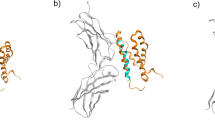
Nutritional metal ions play critical roles in many important immune processes. Hence, the effective modulation of metal ions may open up new forms of immunotherapy, termed as metalloimmunotherapy. Here, we demonstrate a prototype of cancer metalloimmunotherapy using cyclic dinucleotide (CDN) stimulator of interferon genes (STING) agonists and Mn2+. We screened various metal ions and discovered specific metal ions augmented STING agonist activity, wherein Mn2+ promoted a 12- to 77-fold potentiation effect across the prevalent human STING haplotypes. Notably, Mn2+ coordinated with CDN STING agonists to self-assemble into a nanoparticle (CDN–Mn2+ particle, CMP) that effectively delivered STING agonists to immune cells. The CMP, administered either by local intratumoural or systemic intravenous injection, initiated robust anti-tumour immunity, achieving remarkable therapeutic efficacy with minute doses of STING agonists in multiple murine tumour models. Overall, the CMP offers a new platform for local and systemic cancer treatments, and this work underscores the great potential of coordination nanomedicine for metalloimmunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that data supporting the findings of this study are available within the article and its Supplementary Information files. All relevant data can be provided by the authors upon reasonable request.
References
Couzin-Frankel, J. Cancer immunotherapy. Science 342, 1432–1433 (2013).
Gubin, M. M. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581 (2014).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Syn, N. L., Teng, M. W., Mok, T. S. & Soo, R. A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 18, e731–e741 (2017).
Duan, Q., Zhang, H., Zheng, J. & Zhang, L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer 6, 605–618 (2020).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015).
Flood, B. A., Higgs, E. F., Li, S., Luke, J. J. & Gajewski, T. F. STING pathway agonism as a cancer therapeutic. Immunol. Rev. 290, 24–38 (2019).
Shae, D. et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 14, 269–278 (2019).
Schadt, L. et al. Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep. 29, 1236–1248 (2019).
Nicolai, C. J. et al. NK cells mediate clearance of CD8+ T cell-resistant tumors in response to STING agonists. Sci Immunol. https://doi.org/10.1126/sciimmunol.aaz2738 (2020).
Harrington, K. J. et al. Preliminary results of the first-in-human study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with Pembrolizumab (Pembro) in patients with advanced solid tumors or lymphomas. In The European Society for Medical Oncology (ESCO) 2018 Congress Abstract 5475 (2018).
Meric-Bernstam, F. et al. Phase Ib study of MIW815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. J. Clin. Oncol. 37, 2507 (2019).
Ramanjulu, J. M. et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 564, 439–443 (2018).
Chin, E. N. et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science 369, 993–999 (2020).
Pan, B. S. et al. An orally available non-nucleotide STING agonist with antitumor activity. Science https://doi.org/10.1126/science.aba6098 (2020)
Gajewski, T. F. & Higgs, E. F. Immunotherapy with a sting. Science 369, 921–922 (2020).
Koshy, S. T., Cheung, A. S., Gu, L., Graveline, A. R. & Mooney, D. J. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv. Biosyst. https://doi.org/10.1002/adbi.201600013 (2017).
Tan, Y. S. et al. Mitigating SOX2-potentiated immune escape of head and neck squamous cell carcinoma with a STING-inducing nanosatellite vaccine. Clin. Cancer Res. 24, 4242–4255 (2018).
Liu, Y. et al. An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long-term control of lung metastases. Nat. Commun. 10, 5108 (2019).
He, Y. et al. Self-assembled cGAMP-STINGΔTM signaling complex as a bioinspired platform for cGAMP delivery. Sci. Adv. 6, eaba7589 (2020).
Li, S. et al. Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-020-00675-9 (2021).
Chaigne-Delalande, B. & Lenardo, M. J. Divalent cation signaling in immune cells. Trends Immunol. 35, 332–344 (2014).
Wang, C., Zhang, R., Wei, X., Lv, M. & Jiang, Z. Metalloimmunology: the metal ion-controlled immunity. Adv. Immunol. 145, 187–241 (2020).
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Shi, X. et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature 493, 111–115 (2013).
Chandy, K. G. & Norton, R. S. Immunology: channelling potassium to fight cancer. Nature 537, 497–499 (2016).
Vodnala, S. K. et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science https://doi.org/10.1126/science.aau0135 (2019).
Munoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Rossol, M. et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 3, 1329 (2012).
Scambler, T. et al. ENaC-mediated sodium influx exacerbates NLRP3-dependent inflammation in cystic fibrosis. Elife https://doi.org/10.7554/eLife.49248 (2019)
Hood, M. I. & Skaar, E. P. Nutritional immunity: transition metals at the pathogen–host interface. Nat. Rev. Microbiol. 10, 525–537 (2012).
Bessman, N. J. et al. Dendritic cell-derived hepcidin sequesters iron from the microbiota to promote mucosal healing. Science 368, 186–189 (2020).
Wang, C. et al. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 48, 675–687 (2018).
Du, M. & Chen, Z. J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 361, 704–709 (2018).
Chaigne-Delalande, B. et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science 341, 186–191 (2013).
Lv, M. et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 30, 966–979 (2020).
Hou, L. et al. Manganese-based nanoactivator optimizes cancer immunotherapy via enhancing innate immunity. ACS Nano 14, 3927–3940 (2020).
Chen, C. et al. Cytosolic delivery of thiolated Mn-cGAMP nanovaccine to enhance the antitumor immune responses. Small 17, e2006970 (2021).
Yang, X. et al. Converting primary tumor towards an in situ STING-activating vaccine via a biomimetic nanoplatform against recurrent and metastatic tumors. Nano Today 38, 101109 (2021).
Aschner, J. L. & Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 26, 353–362 (2005).
Wang, C. Mangafodipir trisodium (MnDPDP)-enhanced magnetic resonance imaging of the liver and pancreas. Acta Radiol. Suppl. 415, 1–31 (1998).
Takagi, Y. et al. Evaluation of indexes of in vivo manganese status and the optimal intravenous dose for adult patients undergoing home parenteral nutrition. Am. J. Clin. Nutr. 75, 112–118 (2002).
Pan, D., Schmieder, A. H., Wickline, S. A. & Lanza, G. M. Manganese-based MRI contrast agents: past, present and future. Tetrahedron 67, 8431–8444 (2011).
Jin, L. et al. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J. Immunol. 187, 2595–2601 (2011).
Li, L. et al. Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat. Chem. Biol. 10, 1043–1048 (2014).
Thanos, D. & Maniatis, T. Virus induction of human IFNβ gene expression requires the assembly of an enhanceosome. Cell 83, 1091–1100 (1995).
Wang, J. et al. NF-κB RelA subunit is crucial for early IFN-β expression and resistance to RNA virus replication. J. Immunol. 185, 1720–1729 (2010).
Ting, J. P., Duncan, J. A. & Lei, Y. How the noninflammasome NLRs function in the innate immune system. Science 327, 286–290 (2010).
Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630 (2015).
Shin, H. M. et al. Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of NF-κB without affecting IκB degradation. FEBS Lett. 571, 50–54 (2004).
Kuai, R. et al. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 4, eaao1736 (2018).
Sivick, K. E. et al. Magnitude of therapeutic STING activation determines CD8+ T cell-mediated anti-tumor immunity. Cell Rep. 25, 3074–3085 (2018).
Luo, X. et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J. Clin. Invest. 130, 1635–1652 (2020).
Lewis, R. & Tatken, R. Registry of Toxic Effects of Chemical Substances Vol. 1 (US Department of Health and Human Services, National Institute for Occupational Safety and Health, 1980).
Greger, J. L. Nutrition versus toxicology of manganese in humans: evaluation of potential biomarkers. Neurotoxicology 20, 205–212 (1999).
Lutz, M. B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Liu, D., Poon, C., Lu, K., He, C. & Lin, W. Self-assembled nanoscale coordination polymers with trigger release properties for effective anticancer therapy. Nat. Commun. 5, 4182 (2014).
Liu, J. et al. Light-controlled drug release from singlet-oxygen sensitive nanoscale coordination polymers enabling cancer combination therapy. Biomaterials 146, 40–48 (2017).
Yang, Y. et al. One-pot synthesis of pH-responsive charge-switchable PEGylated nanoscale coordination polymers for improved cancer therapy. Biomaterials 156, 121–133 (2018).
Kuai, R. et al. Subcutaneous nanodisc vaccination with neoantigens for combination cancer immunotherapy. Bioconjug. Chem. 29, 771–775 (2018).
Acknowledgements
This work was supported in part by the NIH (R01AI127070, R01CA210273, U01CA210152, R01DK108901, R01DE026728 and R01DE030691), a University of Michigan Rogel Cancer Center Support Grant (P30CA46592) and the University of Michigan, Michigan Drug Discovery (MDD21102). J.J.M. is supported by an NSF CAREER Award (1553831). L.W. was supported in part by the NIH (U24CA232979 and R01CA255242). X. Sun is supported by a Rackham International Student Fellowship and a Rackham Predoctoral Fellowship. We acknowledge J. Hong at the University of Michigan for helping with the ITC analysis, A. Dial at the Michigan Element Analysis Laboratory for Mn biodistribution analysis, K. Chinnaswamy at the University of Michigan Center for Structural Biology for helping with the protein thermal shift assay, J. Whitfield at the University of Michigan Cancer Center Immunology Core for ELISA analysis, H. Carlson at the University of Michigan for molecular dynamic analysis and Q. Zheng at Fujian Medical University Union Hospital for histological analysis. We also thank the University of Michigan Flow Cytometry Core, the ULAM (Unit for Laboratory Animal Medicine) In Vivo Animal Core (IVAC), and the University of Michigan Microscope Imaging Core for technical assistance. We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for the provision of MHC-I tetramers.
Author information
Authors and Affiliations
Contributions
X. Sun, Y.L.L. and J.J.M. designed the experiments. X. Sun performed the experiments. Y.Z., J.L., K.S.P., K.H., X.Z., Y.X., J.N., J.X., X. Shi and L.W. helped with specific experiments. J.L. contributed to the western blotting assays. L.W. and Y.L.L. produced the NOOC1 model and characterized its mutational landscape and response profiles to immunotherapies. Y.X. contributed to the ELISPOT assay. X. Sun, J.L., L.W., Y.L.L. and J.J.M. analysed and interpreted the data. X. Sun, Y.L.L. and J.J.M. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
A patent application (WO2020014644A1) for CMP-based metalloimmunotherapy has been filed, with J.J.M. and X. Sun as inventors. Y.L.L. has licensed the NOOC1 model to Kerafast Inc. (catalogue number: EMU061). The remaining authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Jeffrey Hubbell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods and materials, and Figs. 1–26.
Rights and permissions
About this article
Cite this article
Sun, X., Zhang, Y., Li, J. et al. Amplifying STING activation by cyclic dinucleotide–manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol. 16, 1260–1270 (2021). https://doi.org/10.1038/s41565-021-00962-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-00962-9
This article is cited by
-
Smart responsive Fe/Mn nanovaccine triggers liver cancer immunotherapy via pyroptosis and pyroptosis-boosted cGAS-STING activation
Journal of Nanobiotechnology (2024)
-
Nanomaterial-encapsulated STING agonists for immune modulation in cancer therapy
Biomarker Research (2024)
-
Oncolytic mineralized bacteria as potent locally administered immunotherapeutics
Nature Biomedical Engineering (2024)
-
Immune escape of head and neck cancer mediated by the impaired MHC-I antigen presentation pathway
Oncogene (2024)
-
Promising dawn in tumor microenvironment therapy: engineering oral bacteria
International Journal of Oral Science (2024)