Abstract
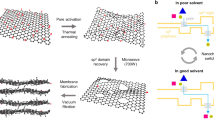
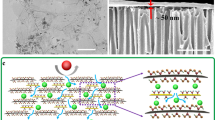
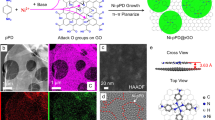
Selective solvent and solute transport across nanopores is fundamental to membrane separations, yet it remains poorly understood, especially for non-aqueous systems. Here, we design a chemically robust nanoporous graphene membrane and study molecular transport in various organic liquids under subnanometre confinement. We show that the nature of the solvent can modulate solute diffusion across graphene nanopores, and that breakdown of continuum flow occurs when pore size approaches the solvent’s smallest molecular cross-section. By holistically engineering membrane support, modelling pore creation and defect management, high rejection and ultrafast organic solvent nanofiltration of dye molecules and separation of hexane isomers are achieved. The membranes exhibit stable fluxes across a range of solvents, consistent with flow across rigid pores whose size is independent of the solvent. These results demonstrate that nanoporous graphene is a rich materials system for controlling subcontinuum flow that could enable new membranes for a range of challenging separation needs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data that support the findings of this study are available in the main text, figures and Supplementary Information. Source data for the main figures are provided with this paper. Source data for Supplementary Figs. 1–19 are available upon reasonable request from the corresponding author.
References
Bocquet, L. & Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 39, 1073–1095 (2010).
Bocquet, L. & Tabeling, P. Physics and technological aspects of nanofluidics. Lab. Chip 14, 3143–3158 (2014).
Bocquet, L. Nanofluidics coming of age. Nat. Mater. 19, 254–256 (2020).
Holt, J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312, 1034–1037 (2006).
Marchetti, P., Jimenez Solomon, M. F., Szekely, G. & Livingston, A. G. Molecular separation with organic solvent nanofiltration: a critical review. Chem. Rev. 114, 10735–10806 (2014).
Chmiola, J. et al. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313, 1760–1763 (2006).
Siria, A. et al. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 494, 455–458 (2013).
Xie, J., Liang, Z. & Lu, Y.-C. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. 19, 1006–1011 (2020).
Grommet, A. B., Feller, M. & Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 15, 256–271 (2020).
Branton, D. et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 26, 1146–1153 (2008).
Kavokine, N., Netz, R. R. & Bocquet, L. Fluids at the nanoscale: from continuum to subcontinuum transport. Annu. Rev. Fluid Mech. 53, 377–410 (2021).
Majumder, M., Chopra, N., Andrews, R. & Hinds, B. J. Enhanced flow in carbon nanotubes. Nature 438, 44 (2005).
Esfandiar, A. et al. Size effect in ion transport through Angstrom-scale slits. Science 358, 511–513 (2017).
Feng, J. et al. Observation of ionic Coulomb blockade in nanopores. Nat. Mater. 15, 850–855 (2016).
Cheng, C., Jiang, G., Simon, G. P., Liu, J. Z. & Li, D. Low-voltage electrostatic modulation of ion diffusion through layered graphene-based nanoporous membranes. Nat. Nanotechnol. 13, 685–690 (2018).
Fumagalli, L. et al. Anomalously low dielectric constant of confined water. Science 360, 1339–1342 (2018).
Mouterde, T. et al. Molecular streaming and its voltage control in Angström-scale channels. Nature 567, 87–90 (2019).
Siria, A., Bocquet, M. L. & Bocquet, L. New avenues for the large-scale harvesting of blue energy. Nat. Rev. Chem. 1, 0091 (2017).
Tang, C. Y., Zhao, Y., Wang, R., Hélix-Nielsen, C. & Fane, A. G. Desalination by biomimetic aquaporin membranes: review of status and prospects. Desalination 308, 34–40 (2013).
Jain, T. et al. Heterogeneous sub-continuum ionic transport in statistically isolated graphene nanopores. Nat. Nanotechnol. 10, 1053–1057 (2015).
Wang, L. et al. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nat. Nanotechnol. 12, 509–522 (2017).
Faucher, S. et al. Critical knowledge gaps in mass transport through single-digit nanopores: a review and perspective. J. Phys. Chem. C 123, 21309–21326 (2019).
Falk, K., Coasne, B., Pellenq, R., Ulm, F. J. & Bocquet, L. Subcontinuum mass transport of condensed hydrocarbons in nanoporous media. Nat. Commun. 6, 6949 (2015).
King, H. E. et al. Pore architecture and connectivity in gas shale. Energy Fuels 29, 1375–1390 (2015).
Vincent, O., Szenicer, A. & Stroock, A. D. Capillarity-driven flows at the continuum limit. Soft Matter 12, 6656–6661 (2016).
Zhong, J. et al. Exploring anomalous fluid behavior at the nanoscale: direct visualization and quantification via nanofluidic devices. Acc. Chem. Res. 53, 347–357 (2020).
Epsztein, R., DuChanois, R. M., Ritt, C. L., Noy, A. & Elimelech, M. Towards single-species selectivity of membranes with subnanometre pores. Nat. Nanotechnol. 15, 426–436 (2020).
Thompson, K. A. et al. N-Aryl-linked spirocyclic polymers for membrane separations of complex hydrocarbon mixtures. Science 369, 310–315 (2020).
Celebi, K. et al. Ultimate permeation across atomically thin porous graphene. Science 344, 289–292 (2014).
Cohen-Tanugi, D. & Grossman, J. C. Water desalination across nanoporous graphene. Nano Lett. 12, 3602–3608 (2012).
Wyss, R. M., Tian, T., Yazda, K., Park, H. G. & Shih, C. J. Macroscopic salt rejection through electrostatically gated nanoporous graphene. Nano Lett. 19, 6400–6409 (2019).
Yang, Y. et al. Large-area graphene-nanomesh/carbon-nanotube hybrid membranes for ionic and molecular nanofiltration. Science 364, 1057–1062 (2019).
Prozorovska, L. & Kidambi, P. R. State-of-the-art and future prospects for atomically thin membranes from 2D materials. Adv. Mater. 30, 1801179 (2018).
Heiranian, M., Farimani, A. B. & Aluru, N. R. Water desalination with a single-layer MoS2 nanopore. Nat. Commun. 6, 8616 (2015).
Thiruraman, J. P., Masih Das, P. & Drndić, M. Stochastic ionic transport in single atomic zero-dimensional pores. ACS Nano 14, 11831–11845 (2020).
Culp, T. E. et al. Nanoscale control of internal inhomogeneity enhances water transport in desalination membranes. Science 371, 72–75 (2021).
Marchena, M. et al. Dry transfer of graphene to dielectrics and flexible substrates using polyimide as a transparent and stable intermediate layer. 2D Mater. 5, 035022 (2018).
Kim, S. et al. Robust graphene wet transfer process through low molecular weight polymethylmethacrylate. Carbon 98, 352–357 (2016).
Boutilier, M. S. et al. Implications of permeation through intrinsic defects in graphene on the design of defect-tolerant membranes for gas separation. ACS Nano 8, 841–849 (2014).
Boutilier, M. S. H. et al. Molecular sieving across centimeter-scale single-layer nanoporous graphene membranes. ACS Nano 11, 5726–5736 (2017).
O’Hern, S. C. et al. Selective molecular transport through intrinsic defects in a single layer of CVD graphene. ACS Nano 6, 10130–10138 (2012).
O’Hern, S. C. et al. Selective ionic transport through tunable subnanometer pores in single-layer graphene membranes. Nano Lett. 14, 1234–1241 (2014).
Karan, S., Jiang, Z. & Livingston, A. G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 348, 1347–1351 (2015).
Yang, Q. et al. Ultrathin graphene-based membrane with precise molecular sieving and ultrafast solvent permeation. Nat. Mater. 16, 1198–1202 (2017).
Gobin, O. C., Reitmeier, S. J., Jentys, A. & Lercher, J. A. Role of the surface modification on the transport of hexane isomers in ZSM-5. J. Phys. Chem. C 115, 1171–1179 (2011).
Funke, H. H., Argo, A. M., Falconer, J. L. & Noble, R. D. Separations of cyclic, branched, and linear hydrocarbon mixtures through silicalite membranes. Ind. Eng. Chem. Res. 36, 137–143 (1997).
Bárcia, P. S., Zapata, F., Silva, J. A. C., Rodrigues, A. E. & Chen, B. Kinetic separation of hexane isomers by fixed-bed adsorption with a microporous metal—organic framework. J. Phys. Chem. B 111, 6101–6103 (2007).
Koh, D. Y., McCool, B. A., Deckman, H. W. & Lively, R. P. Reverse osmosis molecular differentiation of organic liquids using carbon molecular sieve membranes. Science 353, 804–807 (2016).
Heiranian, M., Taqieddin, A. & Aluru, N. R. Revisiting Sampson’s theory for hydrodynamic transport in ultrathin nanopores. Phys. Rev. Res. 2, 043153 (2020).
O’Hern, S. C. et al. Nanofiltration across defect-sealed nanoporous monolayer graphene. Nano Lett. 15, 3254–3260 (2015).
Dong, G. et al. Energy-efficient separation of organic liquids using organosilica membranes via a reverse osmosis route. J. Memb. Sci. 597, 117758 (2020).
Liu, Q. et al. Molecular dynamics simulation of water-ethanol separation through monolayer graphene oxide membranes: significant role of O/C ratio and pore size. Sep. Purif. Technol. 224, 219–226 (2019).
Jang, D., Idrobo, J. C., Laoui, T. & Karnik, R. Water and solute transport governed by tunable pore size distributions in nanoporous graphene membranes. ACS Nano 11, 10042–10052 (2017).
Acknowledgements
This work was funded by Eni S.p.A. through the MIT Energy Initiative. Ion irradiation and SEM imaging were performed at the MRSEC Shared Experimental Facilities supported by the National Science Foundation under award number DMR-0819762 at MIT. We thank S. Carminati, L. Bonoldi, L. Wang, S. Zhang, A. Persad, C. M. Chow and R. Field for valuable discussions.
Author information
Authors and Affiliations
Contributions
R.K. and C.C. conceived the project and designed the research. C.C. fabricated the membranes, performed the imaging characterization, carried out transport measurements and analysed the results. S.A.I. performed the Monte Carlo simulations. All authors were involved in the analysis and discussion of the results. C.C. and R.K. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
C.C. and S.A.I. declare no competing interests. R.K. is coinventor on patents and patent applications on nanoporous graphene membranes.
Additional information
Peer review information Nature Nanotechnology thanks Aleksandra Radenovic, Meni Wanunu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Detailed Materials and Methods, Figs. 1–19 and Tables 1–4.
Source data
Source Data Fig. 2
Statistical source data for solute diffusion across nanoporous atomically thin graphene membranes.
Source Data Fig. 3
Statistical source data for subcontinuum pressure-driven liquid flow through nanoporous atomically thin graphene membranes.
Source Data Fig. 4
Statistical source data for stability of nanoporous atomically thin graphene membranes.
Source Data Fig. 5
Statistical source data for organic solvent nanofiltration with nanoporous atomically thin graphene membranes.
Rights and permissions
About this article
Cite this article
Cheng, C., Iyengar, S.A. & Karnik, R. Molecular size-dependent subcontinuum solvent permeation and ultrafast nanofiltration across nanoporous graphene membranes. Nat. Nanotechnol. 16, 989–995 (2021). https://doi.org/10.1038/s41565-021-00933-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-00933-0
This article is cited by
-
Nanowire-assisted electrochemical perforation of graphene oxide nanosheets for molecular separation
Nature Communications (2024)
-
Bio-inspired design of next-generation ultrapermeable membrane systems
npj Clean Water (2024)
-
Recent advances on liquid intercalation and exfoliation of transition metal dichalcogenides: From fundamentals to applications
Nano Research (2024)
-
High durability and stability of 2D nanofluidic devices for long-term single-molecule sensing
npj 2D Materials and Applications (2023)
-
Cascaded compression of size distribution of nanopores in monolayer graphene
Nature (2023)