Abstract
Extracellular-vesicle-based cell-to-cell communication is conserved across all kingdoms of life. There is compelling evidence that extracellular vesicles are involved in major (patho)physiological processes, including cellular homoeostasis, infection propagation, cancer development and cardiovascular diseases. Various studies suggest that extracellular vesicles have several advantages over conventional synthetic carriers, opening new frontiers for modern drug delivery. Despite extensive research, clinical translation of extracellular-vesicle-based therapies remains challenging. Here, we discuss the uniqueness of extracellular vesicles along with critical design and development steps required to utilize their full potential as drug carriers, including loading methods, in-depth characterization and large-scale manufacturing. We compare the prospects of extracellular vesicles with those of the well established liposomes and provide guidelines to direct the process of developing vesicle-based drug delivery systems.
Similar content being viewed by others
Main
As the field of targeted drug delivery has expanded, nanotechnology has contributed substantially to the development of smart carriers in recent decades1. In particular, lipid-based nanocarriers offer a versatile platform for drug encapsulation, which has led to clinical translation of several formulations. In addition to synthetic nanocarriers, cell-derived extracellular-vesicle (EV)-based carrier systems have attracted considerable interest2.
EVs are a heterogeneous group of small, lipid-bound nanoparticles acting as key mediators of many (patho)physiological processes3. They are also being explored for the delivery of therapeutic payloads to specific cells or tissues, harnessing their intrinsic tissue-homing capabilities4. From a drug delivery perspective, EVs are comparable to liposomes, given that both are phospholipid based. However, EVs are assembled from a complex mixture of various lipids and surface and membrane proteins; some of these components aid tissue targeting, while others ensure minimal non-specific interactions5,6. These unique protein-decorated phospholipid vesicles have been postulated to contain the specific barcodes needed to find their target both locally and at distant sites. Despite extensive research, the superiority of EV-based drug delivery over delivery via engineered nanocarriers, such as liposomes, and the associated risk–benefit ratio remain matters of debate7.
Here, we critically discuss the prospects of EVs as drug delivery vehicles and as next-generation therapeutics. We outline the advantages of EVs over standard delivery methods, discuss current obstacles related to their clinical and industrial translation, and highlight synergies with other emerging fields, such as cell therapeutics (EVs are sometimes considered ‘cell-free cell therapeutics’). We also propose a colour-code guideline regarding experimental requirements and scientific needs to facilitate the development of EVs as drug carriers to evaluate their delivery efficacy and allow benchmarking against alternatives.
Uniqueness of EV biology and function
Composition of EVs
EV secretion appears to be an evolutionarily conserved process present throughout all kingdoms of life8. Regarding fundamental biology, EV research focuses on understanding the biogenesis and release of these natural carriers and their fate upon interaction with target cells. This also comprises the genotypic and phenotypic responses that EVs induce and the mechanisms by which EVs mediate cell-to-cell communication5,9.
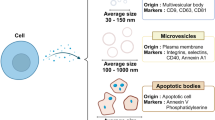
Several subtypes of EV, including exosomes, ectosomes, microvesicles, membrane vesicles and apoptotic bodies, have been identified10. These EVs have been isolated from various sources, including mammalian and prokaryotic cell cultures, blood plasma, bovine milk and plants8. Each EV subpopulation may be derived via distinct biogenesis pathways, and because their precise biogenic origin is impossible to ascertain in most cases, a comprehensive characterization of the vesicles is crucial. In addition, different EV formulations may have substantially different size distributions; thus, standardized characterization is challenging11. The general recommendation in the field is to use ‘EVs’ as a general term. Importantly, the general concept of ‘the EV’ does not exist—currently, the term EV comprises a heterogeneous population, as indicated in the minimal information for studies of extracellular vesicles (MISEV) guidelines12. Proteomic evidence suggests that an EV core protein signature (for example CD63, CD9 or CD81) of highly expressed vesicular proteins is commonly shared between EVs of diverse parent cell origins13. Various tetraspanins are commonly used as molecular markers of EVs. In contrast to the previous MISEV guidelines, there are no typical EV markers that need to be identified on EVs, but careful discrimination of EVs from contaminants, such as protein aggregates and viruses, is important. To add a further layer of complexity, vesicles still carry parent-cell-specific signatures, which are crucial components permitting target-cell interactions in distinctly different manners14. In addition to the core signature of highly expressed and highly enriched vesicular proteins, other typically low-abundance and less enriched protein components are present; these proteins reflect the specific parent-cell origin of the EVs and may also vary depending on the nature and biogenesis of different EV subpopulations15. From a drug delivery perspective, this complexity needs to be understood via comprehensive (multi)omics studies16 and addressed in all characterization and production processes (Fig. 1). In this Review, we elucidate the assembly of EVs on the cellular/molecular level and their mediation of selective intercellular signalling activities to extend biomedical research17.
EVs are produced as heterogeneous mixtures of different subpopulations, and they may participate in proximal and distal communication between cells. After entering the systemic circulation, they must avoid elimination organs, such as the liver, lungs and kidneys, as well as immune cells. Their target-tissue efficiency depends on the degree of functionalization and target-cell interaction.
Uptake and biological role of EVs
Under physiological conditions, EVs are signal carriers involved in the homoeostasis of several processes and of events during cell development, for example cell differentiation18. EV-mediated cross-talk may occur unidirectionally or reciprocally: that is, one cell sends information to another with or without reciprocal signal transmission from the recipient cell, respectively, or even via systemic communication, during which EVs traffic to various tissues and organs. This interaction may involve not only the release and delivery of EV cargo but also cell surface interactions and target-cell modulation, such as immune-cell activation by major histocompatibility complex–peptide interactions. The mechanisms by which EVs are taken up by their target cells are still poorly understood, and examples from the literature are often specific for a certain type of vesicle5. Currently known cellular entry routes of EVs range through receptor-mediated endocytosis, lipid raft interactions, clathrin interactions, phagocytosis, macropinocytosis and possibly direct fusion9. Similarly to many other nanocarriers, EVs taken up into endosomes need to escape the endosomes to release their cargo into the cytosol. Endosomal escape is associated with degradation in acidic compartments of the lysosomal pathway, which could impair the integrity of EV cargoes19. Although EVs were initially postulated to be an unprecedented route for direct cell membrane fusion and cytosolic delivery20, vesicle uptake has been confirmed to be a very complex mechanism, which requires more in-depth evaluation exploring subcellular analyses based on high-resolution microscopy or novel live-cell reporters21. On the other hand, the biological effects induced by EVs are currently well known. During oncogenesis, tumour cells increase their yield of EVs, allowing not only the modulation of surrounding healthy cells, immune cell dysregulation and tumour proliferation but also communication with distant tissues, for example during angiogenesis22. Glioblastoma cells were shown to secrete EVs capable of immunosuppression by blocking T-cell activation and receptor stimulation23. Moreover, widely used cytotoxic drugs, such as taxanes, may also induce shedding of EVs with prometastatic properties24. Although the role of EVs in tumour biology has been investigated extensively, the development of new tools for treatment and diagnostics is still hampered by the absence of tumour-specific EV markers.
A comparable modulatory role of EVs has been observed in the progression of resistance to infections. In the context of viral infections, some EVs may carry viral proteins from infected cells and follow comparable biogenesis pathways25. Furthermore, bacteria utilize EVs for the transmission of resistance genes and virulence factors26, which has sparked interest in the development of bacterial vesicles for vaccination applications27. Bacterial EVs from non-pathogenic or probiotic bacterial sources may also be harnessed as potential EV-based delivery carriers, and their production may be readily scalable by cultivation of EV-producing bacteria in small fermenters28,29. This is a promising avenue for the manufacturing of EVs with novel functionalities and in conjunction with biomaterials30,31. However, immunogenicity requires more detailed evaluation for bacterial vesicles than for mammalian EVs owing to the potential presence of lipopolysaccharides, as recently discussed in detail32.
EV-based drug carriers
Development
With the development of new analytical tools, it has been found that many previously applied isolation techniques are not specific for EVs and lead to the inclusion of contaminants. Methods are constantly refined, but they often expose the limitations in the field, making it difficult for new researchers to follow progress in the state-of-the-art methods. For every drug nanocarrier, a comprehensive physicochemical characterization and its interactions in biological environments must be investigated for therapeutic development. While liposomes have been extensively evaluated for efficacy and biocompatibility both in vitro and in vivo, methodologies well adapted to the considerably more complex EVs are lacking. These natural vesicles are assembled and packaged in a cell-specific manner; for example, cancer-derived EVs carry molecular information distinct from that carried by stem-cell- or blood-cell-derived EVs. While challenging from the perspective of drug carrier development, these properties make EVs a promising biomarker for liquid biopsies in several applications33.
In regenerative medicine, EVs derived from mesenchymal stem cells (MSCs) are already under clinical assessment34 for future use in nanodelivery (Table 1). Stem-cell-derived EVs can induce immune cells to undergo modulation from an activated inflammatory state to a tolerant regulatory state. Some of the strategies used to stimulate EV shedding and enhance yield can also be used for MSCs. N-methyldopamine and norepinephrine induced an increase in MSC-derived EV production without altering their modulatory capacity35. Other approaches apply physical stimuli such as pH variations or low-oxygen conditions, but their long-term effect on the physiological properties of EVs needs to be evaluated. In a murine wound healing model, MSC-EVs were associated with secretion of an interleukin-1 receptor antagonist and induced rapid gingival healing36. Comparable effects have been shown for systemic application of MSC-EVs in patient trials, which has unfortunately led to the use of ‘exosome’ products in unapproved applications. The Food and Drug Administration recently stated that serious adverse effects were experienced by patients in Nebraska treated with unapproved products marketed as containing exosomes37. The agency emphasized that there are currently no regulatory approved EV products and that some clinics “deceive patients with unsubstantiated claims about the potential for these products to prevent, treat or cure various diseases or conditions”. Importantly, any therapeutic application of EVs requires transparent reporting of data on vesicle manufacturing and characterization, suitable quality control provisions, preclinical safety and efficacy38. Moreover, a rational clinical trial design and regulatory monitoring are important to ensure patient safety, as recently indicated by the international societies on stem cells and EVs39. To support the use of MSC-EVs, functional assays that allow in vitro–in vivo correlation of the therapeutic potency of different stem cell preparations must be developed40. Despite these caveats, ongoing efforts to produce EVs from MSCs under Good Manufacturing Process-like conditions41,42 and to design upscaling approaches43 will be instrumental in their development as drug carriers.
Characterization as a prerequisite for meaningful safety and efficacy studies
A comprehensive characterization of EVs and their interaction with cells and tissues is essential for the use of EVs in drug delivery applications. While safety and efficacy characterization is pivotal for the clinical advancement of EVs, insights into the mode of action of EVs may open new frontiers in drug carrier engineering. The identification of critical attributes sufficient to achieve long-distance targeting is crucial to mitigate the risks associated with the high complexity of this system. However, the virus-like size and the increased complexity of EVs compared with synthetic delivery systems (for example liposomes), which partially contribute to the superior drug delivery capacity of EVs, render comprehensive characterization and quality assurance challenging42. Purity and identity issues pose major challenges for analytical techniques, and the inability to characterize the entire system results in substantial risks; these considerations need to be interpreted in the context that EVs constitute a cell-free cell therapy. Standard characterization techniques, for example nanoparticle tracking analysis, imaging flow cytometry and detection of components by biochemical means (including imaging44, flow cytometry and western blotting), involve size measurements. Recently, EVs have also been used as a platform to visualize and study enriched membrane proteins by cryoelectron transmission microscopy45. High-throughput technologies such as next-generation sequencing and mass spectrometry46 (proteomics, lipidomics and transcriptomics), along with cryoelectron microscopy, contribute greatly to the evaluation of the molecular composition and structure of EVs.
Systematic investigation of the efficacy and safety of EVs requires determination of their identity and purity. For this, the International Society for Extracellular Vesicles (ISEV) initiated the standardization of EV isolation and characterization techniques in 2014. The MISEV guidelines were updated in 2018 by 382 researchers12. Box 1 summarizes the most fundamental characteristics that should be evaluated when working with EVs. To further enhance rigour and reproducibility, the EV-TRACK platform was created in 201747. EV-TRACK is a crowdsourcing knowledgebase that allows authors to deposit their isolation and characterization protocols before publication and receive recommendations on potential shortcomings of the experimental design. More recently, additional advice on the optimal reference material for use during EV characterization has been proposed48.
Targeting capabilities and clearance
Liposomes deliver their drug cargo mostly through passive accumulation in certain tissues, unless they carry additional surface ligands. EVs may have an inherent targeting ability and the potential to deliver functional RNA to other cells49 and across certain biological barriers, such as the blood–brain barrier50. For some combinations of parent and target cells, superior tissue-homing capabilities have been identified: for example unidirectional synaptic transfer of microRNA from T cells to antigen-presenting cells51. While synthetic drug delivery systems have shown substantially lower targeting efficacy than natural drug delivery systems, EVs may constitute a natural route for efficient transport52. Indeed, different mammalian tumour EVs were shown to preferentially target healthy cells in the predicted tissue, for example epithelial cells and lung fibroblasts, depending on the integrin expression pattern of the parent cells53. Similar results have been obtained for EVs from sarcoma cells, which showed preferential tumour homing54. For safety reasons, such cancer EVs are not suitable as drug carriers because they may negatively influence tumour invasion or epithelial–mesenchymal transition, or they may carry tumour resistance genes55. A comparative evaluation of EVs derived from different cell lines and their biodistribution pattern showed that, although EVs accumulated primarily in the liver, lung, spleen and gastrointestinal tract, the vesicle source and administration route notably influenced the biodistribution. While dendritic-cell-derived EVs were preferentially taken up by the spleen, melanoma-cell-derived EVs accumulated more prominently in the liver56. Many studies indicate that, similarly to administration of liposomes, systemic EV administration leads to non-specific accumulation in the liver, spleen, gastrointestinal tract and lung56,57. Interestingly, native EVs also showed substantial accumulation in tumour tissue56,57, an effect further enhanced by addition of a specific targeting ligand. However, the half-life of EVs is considerably shorter than that of liposomes. Even when stealth properties were implemented via polyethylene glycol, the terminal half-life of EVs was at most 60 minutes7, while that of modified liposomes could be as long as several hours58. Notably, these studies used fluorescent dyes to label EVs and radionuclides to label liposomes, a difference that may affect comparability. Therefore, more comparative biodistribution studies are required, especially with non-cancer-cell-derived EVs. A head-to-head assessment comparing the delivery efficacy of vesicles and liposomes would also require optimization of the liposomal comparator system in addition to EV engineering59. Another important yet underestimated parameter indicating the efficacy of EV nanocarriers is the mechanical stiffness of the target cell environment. A recent approach using extracellular-matrix-simulating hydrogels showed that EVs were superior to liposomes in escaping stress-relaxing environments60, indicating the influence of vesicle surface proteins.
Immune responses and potential toxicity
Owing to the occurrence of adverse immunological reactions to nanomedicines, such as anaphylaxis, cytokine release syndrome, neutralization of biological activity, cross-reactivity with endogenous protein counterparts and non-acute immune reactions, the production of biologicals has been terminated61. EVs have been widely claimed to be biocompatible on the basis of their mammalian cell origin and ‘physiological’ composition, but such generalization should be avoided. Intravenous administration of EVs purified from bovine milk to mice induced no adverse events and only moderate cytokine release62. Similarly, a wealth of safety data are available on administration of blood-cell-derived EVs during blood transfusions: in most cases without notable adverse effects, even though platelet-derived EVs have most recently been associated with transfusion-related acute lung injury63. In contrast, the potential oncogenic activity of EVs—especially stem-cell-derived EVs with angiogenic activity, which have potential tumour-promoting activity in pre-existing tumours that are dormant due to the lack of angiogenic activity—remains a major concern64. EVs can carry tumour or pathogenic peptides presented by major histocompatibility class receptors to elicit interactions with immune system components, a technique used, for example, in cancer immunotherapy65,66. For carrier development, EVs should have low immunogenicity and be derived from healthy human cells. Intravenous and intraperitoneal administration of EVs derived from human embryonic kidney cells to mice for three weeks did not show toxic effects67. This finding provides an important indication for the potential use of EVs as drug carriers, and almost all data from non-human primate studies are similarly reassuring. In summary, the immunogenicity and biocompatibility of each individual EV formulation must be evaluated, as is the process for liposomal carriers and biologics. Specifically, studies in suitably complex in vitro models, including advanced two- and three-dimensional models68, and their translation to rodent and non-primate animal models, should be evaluated. In addition to organ distribution studies and careful selection of EV labelling methods69, studies evaluating repeated dosing under various regimes are required to allow further clinical investigations.
The use of EVs in an autologous manner by transferring patient cells into culture medium and isolating vesicles for re-administration to the patient has been proposed. Although EVs could be used in this manner for certain applications, several issues limit this strategy, particularly for acute diseases such as infections or cardiovascular incidents70. The autologous use of EVs is feasible under certain conditions, including those for which (1) the use of autologous EVs is important, for example for EV-mediated transfer to mitochondria to maintain genetic compatibility71, and (2) a source of autologous EVs is readily available, for example for the use of blood- or plasma-derived EVs for autologous purposes, such as when vesicles from cancer patients are used in an autologous transplantation protocol aimed at delivering therapeutics to tumour tissues72. However, most applications are likely to use well established non-autologous EVs primarily owing to need, safety considerations and the regulatory/commercial desirability of a streamlined, exceptionally well qualified product. This approach is preferred because non-engineered, non-autologous EVs have been administered to human subjects in numerous clinical studies with good safety outcomes38. Currently, this avenue is being pursued with MSC-EVs for regenerative medicine and EVs derived from dendritic cells for vaccine delivery, as these vesicles were found to be safe in several phase I clinical trials73.
Clinical translation of drug-loaded EVs
While scientists and engineers have attempted to harness the unique properties of EVs to develop smart drug delivery systems that exhibit substantial benefits in targeting, safety and pharmacokinetics compared with those of synthetic nanocarriers, clinical translation of EVs remains challenging74. Owing to the inherent complexity of the EVs themselves, size heterogeneity, and natural (batch-to-batch) variations encountered during their production, the intrinsic risks of the production process are higher than those of purely synthetic production systems.
By appropriate selection and/or engineering of the cells from which EVs are derived, various platforms for loading EVs and conjugating targeting moieties have been developed2. These can be divided into three groups: (1) natural EVs, which are native or obtained from genetically engineered cells; (2) hybrid EVs, which are post-modified with drugs or surface ligands; (3) EV-inspired liposomes (Fig. 2). The active substance in EV-based therapeutics determines their pharmaceutical classification38. From a regulatory perspective, the aforementioned groups probably fall into the pharmaceutical category of biologicals (termed biological medicines, biologicals or biopharmaceuticals, depending on regional practices)38, which includes medicines that contain one or more active substances made by or derived from biological cells. Generally, the active substances in biologicals are more complex than those in non-biological medicines. Currently, substances and constructs with this level of complexity can be produced only by living organisms. However, such production methods are intrinsically faced with a degree of inherent biological variability, which may result in product heterogeneity. From a process design perspective, this heterogeneity is influenced both by biological processes inside the cells used to express the biologicals (upstream processing) and by the manufacturing process used to produce the biologicals (downstream processing)75. Notably, culture conditions, such as the cell passage, cell density and frequency of EV harvesting, strongly influence aspects of product quality, including yield, EV composition and EV bioactivity76. In contrast to mammalian cells produced for cell therapy, for which such deviations may be at least partially compensated by phenotypic adaptations and de novo synthesis, EVs are relatively static products; thus, they are not expected to change substantially after harvesting (with the exception of degradation). Quality-by-design approaches have been proposed for cell-based products. The key tenet is that quality should be built in by design77. While this approach requires an in-depth understanding of the process and the process parameters that need to be evaluated for their association and impact, an analogous approach is appealing for the production of EVs. However, changes in product quality are more challenging to detect and assess in EV production processes than in protein production processes. Overall, because minor changes may have considerable impact on product quality and activity, process stability for EV manufacturing is considered to be lower than that for cell and antibody production. Despite this limitation, EV manufacturing can build on these processes78. While crucial parts of good manufacturing practices can be adapted from the existing fields of biologics, liposomes and cell-based therapies, the size and the compositional and structural complexity of EVs are unique and therefore require additional in-process controls. These in-process controls include measures to ensure that the final product meets the previously identified critical quality attributes and may include measurement of size and concentration, exclusion of contaminants, identification of functional markers and evaluation of drug loading to assess the therapeutic activity per number of vesicles. Due to the size and complexity of EVs, technological limits may hamper the detailed physicochemical, immunochemical or functional characterization of the EV product from an analytical standpoint, specifically from a production and quality control perspective. The currently limited capability for product characterization also has implications for the mechanistic understanding of EV drug delivery products and may render the translational process cumbersome and risky, similar to that of other nanoparticle-based products. However, despite the recent controversy about the potential of nanomedicine79, nanosized formulations permit biodistributions that are different from those possible for free drugs and hence can confer considerable benefits.
Current state
Manufacturers of biologicals are asked to identify all substances in a drug that cause a certain pharmacological, immunological or metabolic action and are responsible for the biological (therapeutic) effects, indicating the mode or mechanism of action of the drug. Additionally, non-active components (termed excipients) used in the final formulation should be declared. Interestingly, in a recent review by the ISEV, it was postulated that, if the whole therapeutic effect could be ascribed to the loaded molecules and not to the EVs in systems with drug-loaded EVs, the EVs would be considered excipients. In this case, only the safety profile and not the mode of action would be requested for the EVs. However, the suitability of conventional toxicity testing approaches for biopharmaceuticals and EVs is limited38 owing to the unique structural and biological properties of these products, such as species specificity, immunogenicity and (unpredicted) pleiotropic activities. Approaches centred on individualized risk analysis, such as those applied to human-cell-based products, may be more applicable, as recently suggested by the ISEV community38.
Scale-up and manufacturing
While EV manufacturing is expected to profit from knowledge in other fields, including the vast experience in protein manufacturing and increasing expertise in cell therapy, the following unit operations are unique to EV production and therefore deserve special attention (Fig. 3).
While upstream processing can adapt safety and quality concepts used in the production of cells for cell therapies, downstream processing can partially adapt concepts used in the production of biologicals. EV isolation and purification are unique and intrinsically prone to viral contamination (sterility issues during upstream and downstream processing are indicated by exclamation marks), which is difficult to remove due to the similar colloidal properties of EVs and viruses and because most purification processes were developed for the purification of viruses.
Key unit operations : upstream
Cell culture and characterization of EV sources
EV manufacturing can profit from developments in classical biologics (that is, antibody and protein production) and cell therapy. Host cell selection and culture conditions constitute critical upstream process steps80. Currently, there is no consensus on the optimal technology for EV production. Parent cells need to be selected on the basis of the activity and tissue-homing properties of EVs and on potential immunogenic and oncogenic considerations. Additionally, genetic stability, host-cell impurities (such as pathogens, and especially viruses) and EV yields play a major role in the parent-cell selection process81. Once selected, these host cells are cultured to efficiently yield EVs with the appropriate phenotype. Suggested methods include multilayered culture flasks, bioreactors and hollow fibre cartridges. For small-scale manufacturing, cells can be expanded in shake flasks, spinners, roller bottles, wave bags or bioreactors. For large-scale cell culture, cells can be grown in stainless steel bioreactors (up to 20,000 l scale), platform-rocker wave bags (up to 500 l scale) or even disposable bioreactors (up to 2,000 l scale)81. From a process safety perspective, closed systems are preferred; however, these systems are more difficult to monitor than open systems42. Genetic drift and contamination need to be monitored closely, following protocols used in the plants producing biologicals and cell therapies. Accumulating evidence indicates that bovine-milk-derived EVs may be a valid alternative source for obtaining large amounts of biocompatible vesicles. Although feasibility studies using milk EVs as drug carriers are underway82, large-scale isolation of pure vesicles from complex milk still needs optimization62.
Optional endogenous drug loading
Parent cells may be engineered to boost EV production and/or yield EVs with enhanced properties. Several methods to load EVs with different molecules have been experimentally evaluated83. These methods include (1) endogenous techniques in which the EV-producing cells also equip vesicles with drug cargo or modified structural protein/RNA components and (2) exogenous approaches in which drugs are loaded into EVs after isolation. The endogenous loading technique has a lower degree of complexity if the producer cells directly shed EVs containing the desired molecule84. Endogenous loading approaches have also been used for the encapsulation of nanoparticle-based drugs into EVs85. Because the loading efficiency is typically limited, cells may be genetically engineered to produce EVs containing the desired active components. However, this approach is limited to biologically accessible drugs, such as nucleic acid and protein drugs. Recently, an optogenetically engineered exosome system that integrates a blue-light responsive membrane module for controllable protein–protein interactions to encapsulate large quantities of anti-inflammatory proteins into EVs was presented86. While highly interesting, genetic engineering of EV-producing cells results in more complex production upscaling than isolation of vesicles from naïve cells and is less elaborate than post-isolation engineering.
EV harvesting and engineering
Product separation and characterization of prepared EVs
For EV isolation, the primary separation of products from cells can be accomplished by well established procedures for the isolation of biologics, including centrifugation, depth filtration (mechanical sieving and adsorption) and tangential flow (cross-flow) filtration. While several strategies for EV isolation and purification, such as differential ultracentrifugation (dUC), precipitation, size exclusion chromatography, affinity chromatography and tangential flow filtration, have been evaluated, there is no consensus on an appropriate EV isolation technique for large-scale manufacturing87. This lack of consensus is mostly because some procedures may negatively affect the integrity and quality of EVs. Additionally, yield and product purity vary among methods and reports, and the overall EV purity generally appears to be low88. Moreover, for heterogeneous EV populations, the isolation method may lead to selective isolation of one specific subpopulation with higher or lower biological activity than the total population. Density gradient centrifugation and dUC offer a decent compromise in terms of yield and purity. dUC is broadly accepted as a common method to obtain ‘pure’ EVs, especially when using chemically defined media without fetal bovine serum. However, careful evaluation of artefacts introduced by the culture medium is pivotal, as it was recently shown that coprecipitation of nucleic acid impurities is possible even under these conditions89. In fact, recent studies suggest that ultrafiltration and size exclusion chromatography (alone or in combination) outperform dUC in terms of both yield and purity90,91. However, depending on the required EV purity, orthogonal methods that target different EV characteristics may need to be integrated at the expense of yield89. Another intrinsic problem arises because most of the currently used purification techniques were originally developed for the purification of viruses42,92, which are amongst the most critical potential contaminants of EVs. Recently, Barone et al. comprehensively analysed the risks, costs and implications of viral contamination in biological manufacturing93. Virus risk mitigation strategies for general biopharmaceutical manufacturing are based on three tenets: (1) prevention of viral entry by selecting low-risk starting and raw materials and using manufacturing controls; (2) testing of in-process materials to ensure they are free of virus and enable lot rejection; (3) clearance of viral contaminants (via inactivation and/or removal) from the product. While both careful selection of cells and raw materials and testing (based on polymerase chain reaction or in vitro virus assays) can reduce risk, the unique size of EVs makes the inactivation and/or removal of viral contaminants even more challenging for EVs than for other biopharmaceutical products. Current strategies are based on affinity rather than size exclusion. Extensive multistep downstream processing may, however, critically force manufacturing costs to increase. In addition to the challenges associated with the complete characterization of contents (including contaminants), the spatial and conformational organization of the constituents is extremely challenging to assess with existing analytical techniques. Even small changes in composition or architecture can strongly affect efficacy and safety. The use of functional activity assays is of utmost importance because isolation methods and the presence of other EV populations or contaminants may decisively influence product activity and off-target effects.
Exogenous drug loading
For clinical translation of EVs, reproducible and technologically accessible methods are needed to load them with the desired drugs94. While loading methods for liposomes have been optimized and applied in industrial production, such settings for EVs are still lacking. Exogenous loading methods work passively by the association of drugs with the lipid bilayer membrane after incubation83, by attaching therapeutics to the EV surface95 and by mechanical or chemical techniques to transiently open the EV membrane to allow diffusion of compounds into the vesicle. The most common approaches to temporarily permeabilize the membrane include sonication, electroporation, saponin treatment and passive incubation96. The advantages and disadvantages of each approach depend on the experimental settings, types of drug and source of EVs, and they can be scaled (Fig. 4c). Passive incubation is a very simple method in which purified EVs are incubated with drugs to allow incorporation into the vesicle membrane. Many early loading protocols followed this method because it exhibits excellent performance for incorporation of hydrophobic compounds such as curcumin97. However, the stability of drugs loaded by passive incorporation across the EV membrane is still unclear. For hydrophilic compounds, loading may be enhanced by the addition of saponin, which has been shown to be effective for large proteins98. Saponins are mild surfactants that induce transient membrane destabilization and may also affect biomolecules; thus, careful purification is needed when saponins are used on a large scale. Mechanical methods for permeabilizing the EV membrane, such as electroporation or sonication, have been shown to be successful for both small molecules and macromolecules50. Even though these methods can be scaled up, their potential influence on protein and nucleic acid drugs requires careful consideration99. In addition to concerns with maintaining the stability of biomacromolecules, the size of the drugs poses another challenge during EV-loading procedures. Large enzymes of >200 kDa have been successfully loaded into EVs using saponin pretreatment98,100. As the size of nucleic acids that may be encapsulated exogenously into EVs is also limited, a cell nanoporation method for large-scale production of functional EVs has been developed101. EV yield and messenger RNA loading were enhanced by this method; however, the required additional steps of transfection and electrical stimulation render its industrial adoption relatively difficult.
a, Consideration of the cell source and associated risks. b, Isolation methods as a function of EV yield and purity. DGC, density gradient centrifugation; SEC, size exclusion chromatography; TFF, tangential flow (cross-flow) filtration. c, Available drug loading methods as a function of loading efficacy and costs for large-scale production109. d, Sizes and densities of potential contaminants and impurities46, which are to be quantitatively detected and removed. HDL, high-density lipoprotein; LDL, low-density lipoprotein; UF, ultrafiltration. e, Methods for formulation and storage of final EV products and the effects of these methods on EV quality110. RT, room temperature. f, Colour-coded guidelines for successful EV-mediated drug delivery. EV development for drug delivery involves the selection of large-scale isolation and characterization, assessment in immunogenicity and biodistribution models, drug loading and functionalization, selection of liposomal comparators, formulation/storage and assessment of batch-to-batch variation. The current state of technology and literary evidence regarding the considerations in each section supporting potential preclinical development is indicated by colour. On the relative scale, high implies comprehensive examples in the literature and substantial technological development, medium indicates the need for further studies based on ongoing efforts and low indicates that more fundamental and comparative studies are required. This diagram indicates that research in no section has reached the highest level and that additional scientific assessments are still required before more standardized and large-scale methods can be developed to bring EV-based carriers closer to clinical translation.
Recently, an alternative method based on liposome fusion has been proposed102. Liposomes containing fusogenic lipids were incubated with EVs, and the cargo of the synthetic liposomes was merged with that from the EVs. Such an approach may pave the way for efficient loading of larger molecules without compromising the EV membrane95.
Downstream
Purification
In addition to the EV isolation and purification steps before drug loading, additional purification steps may be necessary to remove the free drug and exclude the potential contaminants introduced during post-processing (Fig. 4b,d). Magnetic immunoaffinity purification has gained increasing attention owing to the high purity of the obtained products. However, low yields are generally obtained, although theoretical yields may be underestimated due to contaminants.
Quality control—(minimal) characterization of engineered EVs
For routine manufacturing and product monitoring, critical quality attributes need to be defined. Methods to assess these attributes may include evaluations of parent cell properties (for example, evaluation of viability and surface marker expression to assess the phenotype), EV characteristics (for example, evaluation of quantity, size and surface marker expression), assessment of microbial contamination (for example, detection of endotoxin and mycoplasma) and (application-specific) functional activity42. For assessment of batch-to-batch variations, we suggest the use of a concept similar to that applicable to biosimilars—the analytical characteristics of the products should be highly similar to those of the reference product. While non-cell-culture methods (such as isolation from plasma or milk) offer interesting alternatives to cell culture and access to potentially large amounts of EVs, these sources contain EVs originating from many cell types103 that cannot be separated easily. Therefore, characterization of the functional activity of EVs is even more crucial in quantifying on-target and off-target effects.
Formulation and shelf life
Recent studies have focused on the formulation and storage conditions (for example, –80 °C versus 4 °C) of EVs by assessing the size, charge and number of EVs, but strong correlations have not been found95,104. Storage at 4 °C has been shown to cause aggregation and damage to the EV structure105. Moreover, even though the size and number of EVs remained unchanged at −80 °C, alterations in biological activity were detected106. Lyophilization has been investigated as an alternative for long-term storage; however, its impact on vesicle integrity during reconstitution depends on the use of cryoprotectants105. Although storage at −80 °C is recommended, it is the logistically most challenging and costly method (Fig. 4e).
Safety by design and process derisking
While a few years ago the mammalian cell origin of EVs was a major hurdle to their clinical translation, considerable advances have been made in cell-based therapeutics. Regarding safety, EVs derived from autologous cells are associated with lower risks than EVs derived from heterologous cells (including cell lines). However, the time needed to produce autologous EVs is often incompatible with the time available for initiating treatment. During the time required for the manufacturing and quality control of patient-specific EVs, the clinical condition of the patient may worsen, making it impossible to administer the personalized product. Despite the several examples of autologous products developed and commercialized by pharmaceutical companies, the current frameworks seem to be predominantly suited for small-scale academic production rather than for large-scale pharmaceutical production, and production costs may be prohibitive. While the use of allogenic EVs appears generally feasible, the selection of parent cells, assessment of immunologic and oncogenic effects, and risk of viral contamination need to be minimized by continuous monitoring. Selection of assays for monitoring, particularly their sensitivity, is a key challenge in determining the time required for clinical translation of EV-based drug carriers. Regulators have yet to release guidance on how the safety and potency of these EVs should be tested. Currently, EVs are tested batch by batch, with each laboratory and company using different assays107.
Perspectives
EVs may be used as carrier systems for various drug delivery applications. Compared with standard delivery methods, EVs have been shown to deliver functional cargo with decreased immune clearance when administered systemically to rodents. However, more evaluation in clinically relevant systems and direct, quantitative comparison with liposome-based alternatives are required to comprehensively assess the risk–benefit ratio59. Successful translation of EVs depends on the availability of cost-effective large-scale production, isolation and characterization methods with high sensitivity to assess batch-to-batch variations (and their biological consequences), and the availability of widely applicable methods for loading drugs (Fig. 4f). The increasing availability of new analytical techniques is expected to provide new insights into the uniqueness of EVs and may inspire the engineering of next-generation synthetic systems. The production of artificial EVs or EV mimics can overcome challenges related to sterility, mass production and regulation. Exciting new avenues, including the fusion of drug-loaded liposomes with EVs to improve drug loading capabilities, are already being explored102. Notably, the production of designer EVs by implanted cells has recently been reported. This technique offers a new route for in vivo production of engineered exosomes inside the body108. Despite these promising results, more insights into the mechanisms that make EVs so effective at infiltrating cells and evading immune detection are needed to unlock their full potential.
References
van der Meel, R. et al. Smart cancer nanomedicine. Nat. Nanotechnol. 14, 1007–1017 (2019).
Elsharkasy, O. M. et al. Extracellular vesicles as drug delivery systems: why and how? Adv. Drug Deliv. Rev. 159, 332–343 (2020).
Möller, A. & Lobb, R. J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer 20, 697–709 (2020).
El Andaloussi, S., Mager, I., Breakefield, X. O. & Wood, M. J. A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357 (2013).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Hoppstädter, J. et al. Toll-like receptor 2 release by macrophages: an anti-inflammatory program induced by glucocorticoids and lipopolysaccharide. Front. Immunol. 10, 1634 (2019). This original work presented one of the first examples of modifying EVs with polyethylene glycol and the influence on in vivo biodistribution of vesicles.
Kooijmans, S. A. A. et al. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release 224, 77–85 (2016).
Woith, E., Fuhrmann, G. & Melzig, M. F. Extracellular vesicles—connecting kingdoms. Int. J. Mol. Sci. 20, 5695 (2019).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
Witwer, K. W. & Théry, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles 8, 1648167–1648167 (2019).
Cabeza, L. et al. Cancer therapy based on extracellular vesicles as drug delivery vehicles. J. Control. Release 327, 296–315 (2020).
Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
O'Brien, K., Breyne, K., Ughetto, S., Laurent, L. C. & Breakefield, X. O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 21, 585–606 (2020).
Tkach, M., Kowal, J. & Théry, C. Why the need and how to approach the functional diversity of extracellular vesicles. Phil. Trans. R. Soc. B 373, 20160479 (2018).
Hurwitz, S. N. et al. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget 7, 86999–87015 (2016).
Rocha, S. et al. 3D cellular architecture affects microRNA and protein cargo of extracellular vesicles. Adv. Sci. 6, 1800948 (2019).
Fuhrmann, G., Herrmann, I. & Stevens, M. M. Cell-derived vesicles for drug therapy and diagnostics: opportunities and challenges. Nano Today 10, 397–409 (2015).
Gross, J. C., Chaudhary, V., Bartscherer, K. & Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 14, 1036–1045 (2012).
Smith, S. A., Selby, L. I., Johnston, A. P. R. & Such, G. K. The endosomal escape of nanoparticles: toward more efficient cellular delivery. Bioconjugate Chem. 30, 263–272 (2019).
van den Boorn, J. G., Schlee, M., Coch, C. & Hartmann, G. SiRNA delivery with exosome nanoparticles. Nat. Biotechnol. 29, 325–326 (2011).
Sung, B. H. et al. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat. Commun. 11, 2092 (2020).
Xu, R. et al. Extracellular vesicles in cancer—implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 15, 617–638 (2018).
Ricklefs, F. L. et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 4, eaar2766 (2018).
Keklikoglou, I. et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell Biol. 21, 190–202 (2019).
Nolte-‘t Hoen, E., Cremer, T., Gallo, R. C. & Margolis, L. B. Extracellular vesicles and viruses: are they close relatives? Proc. Natl Acad. Sci. USA 113, 9155–9161 (2016).
Toyofuku, M., Nomura, N. & Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 17, 13–24 (2019).
Mehanny, M., Koch, M., Lehr, C.-M. & Fuhrmann, G. Streptococcal extracellular membrane vesicles are rapidly internalized by immune cells and alter their cytokine release. Front. Immunol. 11, 80 (2020).
Goes, A. et al. Myxobacteria-derived outer membrane vesicles: potential applicability against intracellular infections. Cells 9, 194 (2020).
Gujrati, V. et al. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 8, 1525–1537 (2014).
Kuhn, T., Koch, M. & Fuhrmann, G. Probiomimetics—novel lactobacillus-mimicking microparticles show anti-inflammatory and barrier-protecting effects in gastrointestinal models. Small 16, 2003158 (2020).
Murali, V. P. & Holmes, C. A. Biomaterial-based extracellular vesicle delivery for therapeutic applications. Acta Biomater. 124, 88–107 (2021).
Pourtalebi Jahromi, L. & Fuhrmann, G. Bacterial extracellular vesicles: understanding biology promotes applications as nanopharmaceuticals. Adv. Drug Deliv. Rev. 173, 125–140 (2021).
Ayers, L., Pink, R., Carter, D. R. F. & Nieuwland, R. Clinical requirements for extracellular vesicle assays. J. Extracell. Vesicles 8, 1593755 (2019).
Nassar, W. et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 20, 21 (2016).
Wang, J., Bonacquisti, E. E., Brown, A. D. & Nguyen, J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells 9, 660 (2020).
Kou, X. et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci. Transl. Med. 10, eaai8524 (2018).
Public Safety Notification on Exosome Products (FDA, 2019); www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/public-safety-notification-exosome-products
Lener, T. et al. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J. Extracell. Vesicles 4, 30087 (2015). A seminal position paper from an international consortium of EV scientists on the regulatory needs when studying vesicles in clinical trials.
Börger, V. et al. International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy 22, 482–485 (2020).
Galipeau, J. The mesenchymal stromal cells dilemma—does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 15, 2–8 (2013).
Witwer, K. W. et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 8, 1609206 (2019).
Rohde, E., Pachler, K. & Gimona, M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy 21, 581–592 (2019). This perspective provides a roadmap for the development of EV-based therapeutics in a very early stage of manufacturing as well as during early clinical safety and proof-of-concept testing.
Zipkin, M. Exosome redux. Nat. Biotechnol. 37, 1395–1400 (2019).
Chuo, S. T.-Y., Chien, J. C.-Y. & Lai, C. P.-K. Imaging extracellular vesicles: current and emerging methods. J. Biomed. Sci. 25, 91 (2018).
Zeev-Ben-Mordehai, T., Vasishtan, D., Siebert, C. A., Whittle, C. & Grünewald, K. Extracellular vesicles: a platform for the structure determination of membrane proteins by cryo-EM. Structure 22, 1687–1692 (2014).
Kreimer, S. et al. Mass-spectrometry-based molecular characterization of extracellular vesicles: lipidomics and proteomics. J. Proteome Res 14, 2367–2384 (2015).
Van Deun, J. et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 14, 228–232 (2017).
Welsh, J. A. et al. Towards defining reference materials for measuring extracellular vesicle refractive index, epitope abundance, size and concentration. J. Extracell. Vesicles 9, 1816641 (2020). This paper provides guidelines on the standardization of commonly used analysis platforms for characterizing EV refractive index, epitope abundance, size and concentration.
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Mittelbrunn, M. et al. Unidirectional transfer of micro RNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2, 282 (2011).
Murphy, D. E. et al. Natural or synthetic RNA delivery: a stoichiometric comparison of extracellular vesicles and synthetic nanoparticles. Nano Lett. 21, 1888–1895 (2021).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Qiao, L. et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 10, 3474–3487 (2020).
Dai, J. et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 5, 145 (2020).
Wiklander, O. P. B. et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4, 26316 (2015).
Lai, C. P. et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8, 483–494 (2014).
Henriksen, J. R. et al. Remote loading of 64Cu2+ into liposomes without the use of ion transport enhancers. ACS Appl. Mater. Interfaces 7, 22796–22806 (2015).
Johnsen, K. B. et al. On the use of liposome controls in studies investigating the clinical potential of extracellular vesicle-based drug delivery systems—a commentary. J. Control. Release 269, 10–14 (2018).
Lenzini, S., Bargi, R., Chung, G. & Shin, J.-W. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 15, 217–223 (2020). In this recent work, the influence of mechanical properties of EVs on the diffusion from extracellular-matrix-simulating environments was evaluated.
Geigert, J. in The Challenge of CMC Regulatory Compliance for Biopharmaceuticals and Other Biologics (ed. Geigert, J.) 221–237 (Springer, 2013).
Somiya, M., Yoshioka, Y. & Ochiya, T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles 7, 1440132 (2018).
McVey, M. J. et al. Platelet extracellular vesicles mediate transfusion-related acute lung injury by imbalancing the sphingolipid rheostat. Blood 137, 690–701 (2021).
Balachandran, B. & Yuana, Y. Extracellular vesicles-based drug delivery system for cancer treatment. Cogent Med. 6, 1635806 (2019).
Robbins, P. D. & Morelli, A. E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14, 195–208 (2014).
Mehanny, M., Lehr, C.-M. & Fuhrmann, G. Extracellular vesicles as antigen carriers for novel vaccination avenues. Adv. Drug Deliv. Rev. 173, 164–180 (2021).
Zhu, X. et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 6, 1324730 (2017).
Thippabhotla, S., Zhong, C. & He, M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci. Rep. 9, 13012 (2019).
Li, Y.-J. et al. Emerging strategies for labeling and tracking of extracellular vesicles. J. Control. Release 328, 141–159 (2020).
de Abreu, R. C. et al. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol. 17, 685–697 (2020).
Ikeda, G. et al. Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardium. J. Am. Coll. Cardiol. 77, 1073–1088 (2021).
Villa, A. et al. Transplantation of autologous extracellular vesicles for cancer-specific targeting. Theranostics 11, 2034–2047 (2021).
Escudier, B. et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J. Transl. Med. 3, 10 (2005).
Clemmens, H. & Lambert, D. W. Extracellular vesicles: translational challenges and opportunities. Biochem. Soc. Trans. 46, 1073–1082 (2018).
Vulto, A. G. & Jaquez, O. A. The process defines the product: what really matters in biosimilar design and production? Rheumatology 56, iv14–iv29 (2017).
Patel, D. B. et al. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng. Transl. Med. 2, 170–179 (2017).
Silverman, L. I. et al. Identifying and managing sources of variability in cell therapy manufacturing and clinical trials. Regen. Eng. Transl. Med. 5, 354–361 (2019).
Iancu, E. M. & Kandalaft, L. E. Challenges and advantages of cell therapy manufacturing under Good Manufacturing Practices within the hospital setting. Curr. Opin. Biotechnol. 65, 233–241 (2020).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Geigert, J. in The Challenge of CMC Regulatory Compliance for Biopharmaceuticals and Other Biologics (ed. Geigert, J.) 105–137 (Springer, 2013).
Geigert, J. in The Challenge of CMC Regulatory Compliance for Biopharmaceuticals and Other Biologics (ed. Geigert, J.) 139–178 (Springer, 2013).
Munagala, R., Aqil, F., Jeyabalan, J. & Gupta, R. C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 371, 48–61 (2016).
Fuhrmann, G., Serio, A., Mazo, M., Nair, R. & Stevens, M. M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 205, 35–44 (2015). One of the first comprehensive examples of comparing different loading methods for various types of EV using a set of model compounds with varying water solubility.
Schulz, E. et al. Biocompatible bacteria-derived vesicles show inherent antimicrobial activity. J. Control. Release 290, 46–55 (2018).
Piffoux, M. et al. Extracellular vesicle production loaded with nanoparticles and drugs in a trade-off between loading, yield and purity: towards a personalized drug delivery system. Adv. Biosyst. 1, 1700044 (2017).
Choi, H. et al. Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality. Sci. Adv. 6, eaaz6980 (2020).
Meng, W. et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 27, 585–598 (2020).
Lee, Y. X. F., Johansson, H., Wood, M. J. A. & El Andaloussi, S. Considerations and implications in the purification of extracellular vesicles—a cautionary tale. Front. Neurosci. 13, 1067 (2019).
Auber, M., Fröhlich, D., Drechsel, O., Karaulanov, E. & Krämer-Albers, E.-M. Serum-free media supplements carry miRNAs that co-purify with extracellular vesicles. J. Extracell. Vesicles 8, 1656042 (2019).
Nordin, J. Z. et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine 11, 879–883 (2015).
Benedikter, B. J. et al. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci. Rep. 7, 15297 (2017).
Corso, G. et al. Reproducible and scalable purification of extracellular vesicles using combined bind–elute and size exclusion chromatography. Sci. Rep. 7, 11561 (2017).
Barone, P. W. et al. Viral contamination in biologic manufacture and implications for emerging therapies. Nat. Biotechnol. 38, 563–572 (2020).
Armstrong, J. P. K. & Stevens, M. M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 130, 12–16 (2018).
de Jong, O. G. et al. Drug delivery with extracellular vesicles: from imagination to innovation. Acc. Chem. Res. 52, 1761–1770 (2019).
Luan, X. et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 38, 754–763 (2017).
Sun, D. et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 18, 1606–1614 (2010).
Haney, M. J. et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 207, 18–30 (2015).
Kooijmans, S. A. A. et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 172, 229–238 (2013).
Fuhrmann, G. et al. Engineering extracellular vesicles with the tools of enzyme prodrug therapy. Adv. Mater. 30, 1706616 (2018).
Yang, Z. et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 4, 69–83 (2020).
Piffoux, M., Silva, A. K. A., Wilhelm, C., Gazeau, F. & Tareste, D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano 12, 6830–6842 (2018). This work gives a well studied example of controlled fusion of EVs with liposomes to enhance loading and surface functionalization of the vesicles.
Hartjes, T. A., Mytnyk, S., Jenster, G. W., van Steijn, V. & van Royen, M. E. Extracellular vesicle quantification and characterization: common methods and emerging approaches. Bioengineering 6, 7 (2019).
Deville, S. et al. Comparison of extracellular vesicle isolation and storage methods using high-sensitivity flow cytometry. PLoS ONE 16, e0245835 (2021).
Paganini, C. et al. Scalable production and isolation of extracellular vesicles: available sources and lessons from current industrial bioprocesses. Biotechnol. J. 14, 1800528 (2019).
Lőrincz, Á. M. et al. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J. Extracell. Vesicles 3, 25465–25465 (2014).
Keener, A. B. How extracellular vesicles can enhance drug delivery. Nature 582, S14–S15 (2020).
Kojima, R. et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 9, 1305 (2018).
Rufino-Ramos, D. et al. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J. Control. Release 262, 247–258 (2017).
Richter, M., Fuhrmann, K. & Fuhrmann, G. Evaluation of the storage stability of extracellular vesicles. J. Vis. Exp. (147), e59584 (2019).
Acknowledgements
I.K.H. acknowledges support from the Swiss National Science Foundation (grant 181290). G.F. acknowledges funding from the Federal Ministry for Research and Education (grant 13XP5029A, NanoMatFutur programme).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing interests: M.J.A.W. is a cofounder and shareholder of Evox Therapeutics.
Additional information
Peer review information Nature Nanotechnology thanks Mansoor Amiji and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Herrmann, I.K., Wood, M.J.A. & Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16, 748–759 (2021). https://doi.org/10.1038/s41565-021-00931-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-00931-2
This article is cited by
-
Peripheral extracellular vesicles in neurodegeneration: pathogenic influencers and therapeutic vehicles
Journal of Nanobiotechnology (2024)
-
Co-culture engineering: a promising strategy for production of engineered extracellular vesicle for osteoarthritis treatment
Cell Communication and Signaling (2024)
-
Nanomaterial-encapsulated STING agonists for immune modulation in cancer therapy
Biomarker Research (2024)
-
Synergistic vesicle-vector systems for targeted delivery
Journal of Nanobiotechnology (2024)
-
Extracellular vesicles involved in growth regulation and metabolic modulation in Haematococcus pluvialis
Biotechnology for Biofuels and Bioproducts (2024)