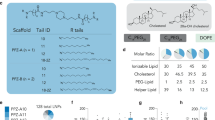
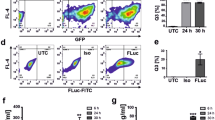
Abstract
The successful in vivo implementation of gene expression modulation strategies relies on effective, non-immunogenic delivery vehicles. Lipid nanoparticles are one of the most advanced non-viral clinically approved nucleic-acid delivery systems. Yet lipid nanoparticles accumulate naturally in liver cells upon intravenous administration, and hence, there is an urgent need to enhance uptake by other cell types. Here we use a conformation-sensitive targeting strategy to achieve in vivo gene silencing in a selective subset of leukocytes and show potential therapeutic applications in a murine model of colitis. In particular, by targeting the high-affinity conformation of α4β7 integrin, which is a hallmark of inflammatory gut-homing leukocytes, we silenced interferon-γ in the gut, resulting in an improved therapeutic outcome in experimental colitis. The lipid nanoparticles did not induce adverse immune activation or liver toxicity. These results suggest that our lipid nanoparticle targeting strategy might be applied for selective delivery of payloads to other conformation-sensitive targets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data are available from the authors upon reasonable request.
References
Elinav, E. & Peer, D. Harnessing nanomedicine for mucosal theranostics—a silver bullet at last? ACS Nano 7, 2883–2890 (2013).
Hanauer, S. B. et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2, 542–553 (2004).
Baert, F. et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N. Engl. J. Med. 348, 601–608 (2003).
Kedmi, R. et al. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 13, 214–219 (2018).
Veiga, N. et al. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 9, 4493 (2018).
Whitehead, K. A., Langer, R. & Anderson, D. G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 8, 129–138 (2009).
Fenske, D. B., Chonn, A. & Cullis, P. R. Liposomal nanomedicines: an emerging field. Toxicol. Pathol. 36, 21–29 (2008).
Ledford, H. Gene silencing technology gets first drug approval after 20-year wait. Nature 560, 291–292 (2018).
Ramishetti, S. et al. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano 9, 6706–6716 (2015).
Peer, D., Park, E. J., Morishita, Y., Carman, C. V. & Shimaoka, M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319, 627–630 (2008).
Meenan, J. et al. Altered expression of α4β7, a gut homing integrin, by circulating and mucosal T cells in colonic mucosal inflammation. Gut 40, 241–246 (1997).
Yu, Y. et al. Structural specializations of α4β7, an integrin that mediates rolling adhesion. J. Cell Biol. 196, 131–146 (2012).
Sun, H. et al. Distinct chemokine signaling regulates integrin ligand specificity to dictate tissue-specific lymphocyte homing. Dev. Cell 30, 61–70 (2014).
Lichtenstein, G. R., Hanauer, S. B. & Sandborn, W. J. Risk of biologic therapy-associated progressive multifocal leukoencephalopathy: use of the JC virus antibody assay in the treatment of moderate-to-severe Crohn’s disease. Gastroenterol. Hepatol. 8, 1–20 (2012).
Green, N. et al. Mutational analysis of MAdCAM-1/α4β7 interactions reveals significant binding determinants in both the first and second immunuglobulin domains. Cell Adhes. Commun. 7, 167–181 (1999).
Shyjan, A. M., Bertagnolli, M., Kenney, C. J. & Briskin, M. J. Human mucosal addressin cell adhesion molecule-1 (MAdCAM-1) demonstrates structural and functional similarities to the α4β7-integrin binding domains of murine MAdCAM-1, but extreme divergence of mucin-like sequences. J. Immunol. 156, 2851–2857 (1996).
Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Dearling, J. L. J. et al. Detection of intestinal inflammation by MicroPET imaging using a 64Cu-labeled anti-β7 integrin antibody. Inflamm. Bowel Dis. 16, 1458–1466 (2010).
Dearling, J. L. J., Daka, A., Veiga, N., Peer, D. & Packard, A. B. Colitis immunoPET: defining target cell populations and optimizing pharmacokinetics. Inflamm. Bowel Dis. 22, 529–538 (2016).
Belliveau, N. M. et al. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther. Nucleic Acids 1, e37 (2012).
Cohen, Z. R. et al. Localized RNAi therapeutics of chemoresistant grade IV glioma using hyaluronan-grafted lipid-based nanoparticles. ACS Nano 9, 1581–1591 (2015).
Veiga, N. et al. Leukocyte-specific siRNA delivery revealing IRF8 as a potential anti-inflammatory target. J. Control Release 313, 33–41 (2019).
Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. & Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9, 1410 (2018).
Chassaing, B., Aitken, J. D., Malleshappa, M. & Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 104, 15.25.1–15.25.14 (2014).
Dieleman, L. A. et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107, 1643–1652 (1994).
Berg, D. J. et al. Rapid development of colitis in NSAID-treated IL-10 deficient mice. Gastroenterology 123, 1527–1542 (2002).
Holgersen, K., Kvist, P. H., Markholst, H., Kornerup Hansen, A. & Holm, T. L. Characterisation of enterocolitis in the piroxicam-accelerated interleukin-10 knock out mouse—a model mimicking inflammatory bowel disease J. Crohn’s Colitis 8, 147–160 (2012).
Holgersen, K., Kvist, P. H., Hansen, A. K. & Holm, T. L. Predictive validity and immune cell involvement in the pathogenesis of piroxicam-accelerated colitis in interleukin-10 knockout mice. Int. Immunopharmacol. 21, 137–147 (2014).
Connor, E. M., Eppihimer, M. J., Morise, Z., Granger, D. N. & Grisham, M. B. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J. Leukoc. Biol. 65, 349–355 (1999).
Ito, R. et al. Interferon-γ is causatively involved in experimental inflammatory bowel disease in mice. Clin. Exp. Immunol. 146, 330–338 (2006).
Ferreiro, R. & Barreiro-de Acosta, M. Infliximab: Pharmacology, Uses and Limitations 1st edn (eds Acevedo, A. D. M. & Gaitan, M. F.) 39–74 (Nova Biomedical, 2012).
Vila-del Sol, V., Punzón, C. & Fresno, M. IFN-γ-induced TNF-α expression is regulated by interferon regulatory factors 1 and 8 in mouse macrophages. J. Immunol. 181, 4461–4470 (2008).
Wesemann, D. R. & Benveniste, E. N. STAT-1α and IFN-γ as modulators of TNF-α signaling in macrophages: regulation and functional implications of the TNF receptor 1:STAT-1α complex. J. Immunol. 171, 5313–5319 (2003).
Shimaoka, M. et al. AL-57, a ligand-mimetic antibody to integrin LFA-1, reveals chemokine-induced affinity up-regulation in lymphocytes. Proc. Natl Acad. Sci. USA 103, 13991–13996 (2006).
Qi, J. P. et al. Identification, characterization, and epitope mapping of human monoclonal antibody J19 that specifically recognizes activated integrin α4β7. J. Biol. Chem. 287, 15749–15759 (2012).
Kinashi, T. Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 5, 546–559 (2005).
Eun, J. P. et al. Aberrant activation of integrin α4β7suppresses lymphocyte migration to the gut. J. Clin. Invest. 117, 2526–2538 (2007).
YANG, Y. et al. Construction and adhesive properties of a soluble MAdCAM‐1–Fc chimera expressed in a baculovirus system: phylogenetic conservation of receptor–ligand interaction. Scand. J. Immunol. 42, 235–247 (1995).
Rungta, R. L. et al. Lipid nanoparticle delivery of siRNA to silence neuronal gene expression in the brain. Mol. Ther. Nucleic Acids 2, e136 (2013).
McCall, M. J., Diril, H. & Meares, C. F. Simplified method for conjugating macrocyclic bifunctional chelating agents to antibodies via 2-iminothiolane. Bioconjugate Chem. 1, 222–226 (1990).
Loening, A. M. & Gambhir, S. S. AMIDE: a free software tool for multimodality medical image analysis. Mol. Imaging 2, 131–137 (2003).
Acknowledgements
We thank V. Holdengreber for assistance with the transmission electron microscopy analysis, P. Johnston for detailed statistical analysis of the molecular imaging part, S. Chatterjee for help with the confocal microscope and S. Jung for providing the IL-10KO mice. This work was supported by the ERC grant LeukoTheranostics (award no. 647410) to D.P.
Author information
Authors and Affiliations
Contributions
N.D. and D.P. conceived the study. N.D., S.R., N.V., M.G. and J.L.J.D. performed the experiments. N.D., J.L.J.D., A.B.P., M.G. and D.P. analysed the data. N.D. and D.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.P. receives licensing fees (to patents on which he was an inventor) from, invested in, consults (or on scientific advisory boards or boards of directors) for, lectured (and received a fee) or conducts sponsored research at TAU for the following entities: Alnylam Pharmaceuticals Inc. Arix Biosciences Inc., ART Biosciences, BioNtech RNA pharmaceuticals; Centricus, Diagnostear Ltd., EPM Inc., Earli Inc., lmpetis Biosciences, Kernal Biologics, GPCR Inc., Medison Pharma Ltd., Newphase Ltd, NLC Pharma Ltd., Nanocell Therapeutics, NanoGhosts Ltd., Precision Nanosystems Inc., Paul Hastings Inc., Regulon, Roche, SciCann, Shire Inc., VLX Ventures, TATA Cooperation, Teva Pharmaceuticals Inc., Wize Pharma Ltd. All other authors declare no competing financial interests. None of them relates to this work. The rest of the authors declare no financial interests.
Additional information
Peer review information Nature Nanotechnology thanks Monica Baiula, JianFeng Chen, Yizhou Dong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10.
Rights and permissions
About this article
Cite this article
Dammes, N., Goldsmith, M., Ramishetti, S. et al. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. Nat. Nanotechnol. 16, 1030–1038 (2021). https://doi.org/10.1038/s41565-021-00928-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-00928-x
This article is cited by
-
Targeted delivery of Fc-fused PD-L1 for effective management of acute and chronic colitis
Nature Communications (2024)
-
Natural long-chain saturated fatty acids doped LNPs enabling spleen selective mRNA translation and potent cancer immunotherapy
Nano Research (2024)
-
Dual peptides-modified cationic liposomes for enhanced Lung cancer gene therapy by a gap junction regulating strategy
Journal of Nanobiotechnology (2023)
-
Integrin signaling in cancer: bidirectional mechanisms and therapeutic opportunities
Cell Communication and Signaling (2023)
-
Targeted modulation of immune cells and tissues using engineered biomaterials
Nature Reviews Bioengineering (2023)