Abstract
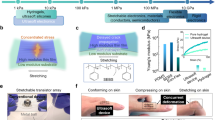
Living tissues are non-linearly elastic materials that exhibit viscoelasticity and plasticity. Man-made, implantable bioelectronic arrays mainly rely on rigid or elastic encapsulation materials and stiff films of ductile metals that can be manipulated with microscopic precision to offer reliable electrical properties. In this study, we have engineered a surface microelectrode array that replaces the traditional encapsulation and conductive components with viscoelastic materials. Our array overcomes previous limitations in matching the stiffness and relaxation behaviour of soft biological tissues by using hydrogels as the outer layers. We have introduced a hydrogel-based conductor made from an ionically conductive alginate matrix enhanced with carbon nanomaterials, which provide electrical percolation even at low loading fractions. Our combination of conducting and insulating viscoelastic materials, with top-down manufacturing, allows for the fabrication of electrode arrays compatible with standard electrophysiology platforms. Our arrays intimately conform to the convoluted surface of the heart or brain cortex and offer promising bioengineering applications for recording and stimulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Code availability
The code associated with the work of this manuscript is available at https://doi.org/10.7910/DVN/8K77QG (ref. 43).
References
Tolstosheeva, E. et al. A multi-channel, flex-rigid ECoG microelectrode array for visual cortical interfacing. Sensors (Basel) 15, 832–854 (2015).
Luan, L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 3, e1601966 (2017).
Tybrandt, K. et al. High-density stretchable electrode grids for chronic neural recording. Adv. Mater. 30, e1706520 (2018).
Konerding, W. S., Froriep, U. P., Kral, A. & Baumhoff, P. New thin-film surface electrode array enables brain mapping with high spatial acuity in rodents. Sci. Rep. 8, 1–14 (2018).
Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 1, 16063 (2016).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Chaudhuri, O. et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 13, 970–978 (2014).
Budday, S. et al. Region- and loading-specific finite viscoelasticity of human brain tissue. Proc. Appl. Math. Mech. 18, 3–4 (2018).
Wang, Z., Golob, M. J. & Chesler, N. C. in Viscoelastic and Viscoplastic Materials (InTech: 2016); https://doi.org/10.5772/64169
Boyle, N. G. & Shivkumar, K. Epicardial interventions in electrophysiology. Circulation 126, 1752–1769 (2012).
Chaudhuri, O. et al. Substrate stress relaxation regulates cell spreading. Nat. Commun. 6, 6365 (2015).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Rubehn, B., Bosman, C., Oostenveld, R., Fries, P. & Stieglitz, T. A MEMS-based flexible multichannel ECoG-electrode array. J. Neural Eng. 6, 036003 (2009).
Bramini, M. et al. Interfacing graphene-based materials with neural cells. Front. Syst. Neurosci. 12, https://doi.org/10.3389/fnsys.2018.00012 (2018).
Kostarelos, K. & Novoselov, K. S. Graphene devices for life. Nat. Nanotechnol. 9, 744–745 (2014).
Martín, C., Kostarelos, K., Prato, M. & Bianco, A. Biocompatibility and biodegradability of 2D materials: graphene and beyond. Chem. Commun. 55, 5540–5546 (2019).
Pampaloni, N. P., Giugliano, M., Scaini, D., Ballerini, L. & Rauti, R. Advances in nano neuroscience: from nanomaterials to nanotools. Front. Neurosci. 12, 953 (2019).
Lu, B. et al. Pure PEDOT:PSS hydrogels. Nat. Commun. 10, 1043 (2019).
Liu, Y. et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 38, 1031–1036 (2020).
Yuan, X., Wei, Y., Chen, S., Wang, P. & Liu, L. Bio-based graphene/sodium alginate aerogels for strain sensors. RSC Adv. 6, 64056–64064 (2016).
Golafshan, N., Kharaziha, M. & Fathi, M. Tough and conductive hybrid graphene-PVA: alginate fibrous scaffolds for engineering neural construct. Carbon 111, 752–763 (2017).
Lin, X. et al. A viscoelastic adhesive epicardial patch for treating myocardial infarction. Nat. Biomed. Eng. 3, 632–643 (2019).
Son, D. et al. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol. 13, 1057–1065 (2018).
Masvidal-Codina, E. et al. High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors. Nat. Mater. 18, 280–288 (2019).
Green, R. Elastic and conductive hydrogel electrodes. Nat. Biomed. Eng. 3, 9–10 (2019).
Choi, S. et al. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 13, 1048–1056 (2018).
Lee, S. et al. Ultrasoft electronics to monitor dynamically pulsing cardiomyocytes. Nat. Nanotechnol. 14, 156–160 (2019).
Lee, K. Y. & Mooney, D. J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126 (2012).
Sun, J. Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Kang, J. et al. Tough and water-insensitive self-healing elastomer for robust electronic skin. Adv. Mater. 30, e1706846 (2018).
Tondera, C. et al. Highly conductive, stretchable, and cell-adhesive hydrogel by nanoclay doping. Small 15, 1901406 (2019).
Kim, N. et al. Elastic conducting polymer composites in thermoelectric modules. Nat. Commun. 11, 1424 (2020).
Wang, Y. et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 3, e1602076 (2017).
Feig, V. R., Tran, H., Lee, M. & Bao, Z. Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat. Commun. 9, 2740 (2018).
Vicente, J., Costa, P., Lanceros-Mendez, S., Abete, J. M. & Iturrospe, A. Electromechanical properties of PVDF-based polymers reinforced with nanocarbonaceous fillers for pressure sensing applications. Materials (Basel) 12, 3545 (2019).
Chen, Z. et al. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 10, 424–428 (2011).
Bhagavatheswaran, E. S. et al. Construction of an interconnected nanostructured carbon black network: development of highly stretchable and robust elastomeric conductors. J. Phys. Chem. C. 119, 21723–21731 (2015).
Haggenmueller, R., Gommans, H. H., Rinzler, A. G., Fischer, J. E. & Winey, K. I. Aligned single-wall carbon nanotubes in composites by melt processing methods. Chem. Phys. Lett. 330, 219–225 (2000).
Chen, Y. et al. A skin-inspired stretchable, self-healing and electro-conductive hydrogel with a synergistic triple network for wearable strain sensors applied in human-motion detection. Nanomaterials 9, 1737 (2019).
Doney, E. et al. 3D printing of preclinical X-ray computed tomographic data sets. J. Vis. Exp. 22, e50250 (2013).
Rowley, J. A., Madlambayan, G. & Mooney, D. J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20, 45–53 (1999).
Topsoe, H. Geometric Factors in Four Point Resistivity Measurement. Bulletin No. 472-13 (IISERKOL, 1966). http://four-point-probes.com/haldor-topsoe-geometric-factors-in-four-point-resistivity-measurement/
Tringides, C. & Vachicouras, N. Impedance spectra and cyclic voltammetry analysis. Harvard Dataverse, V1 (2021); https://doi.org/10.7910/DVN/8K77QG
Acknowledgements
The authors thank T. Sirota and P. Machado, both at the Wyss Institute, Boston Massachusetts, for their help with 3D printing and the machining of moulds, respectively. This work was supported in part by the Center for Nanoscale Systems at Harvard University, which is a member of the National Nanotechnology Infrastructure Network, which is supported by the National Science Foundation under award no. 1541959. We thank the Weitz lab for the use of their rheometer, which is funded by the Materials Research Science and Engineering Center of Harvard University under National Science Foundation award no. DMR 14-20570. This work was supported by an NSF GRFP to C.M.T., as well as funding for C.M.T. through an NIH grant awarded to D.J.M. (RO1DE013033), NSF MRSEC award DMR 14-20570 and funding by the Wyss Institute for Biologically Inspired Engineering at Harvard University. I.d.L. was supported by the National Cancer Institute of the National Institutes of Health under award no. U01CA214369. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. H.W. gratefully acknowledges funding support from the Wyss Technology Development Fellowship. B.R.S. is supported by the National Institute of Dental and Craniofacial Research (R01DE013349) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P2CHD086843). A.E.-A. received funding for this work from the European Union’s Horizon 2020 research and innovation programme through a Marie Sklodowska-Curie grant agreement no. 798504 (MECHANOSITY). K.K., C.C. and Y.S. were mainly funded by the EPSRC Programme Grant 2D-Health (EP/P00119X/1). C.C. acknowledges support by the EPSRC (EP/N010345/1). N.V., A.T., F.F. and S.P.L. were funded by the Bertarelli Foundation, the Wyss Center Geneva and SNSF Sinergia grant no. CRSII5_183519.
Author information
Authors and Affiliations
Contributions
C.M.T. and D.J.M. conceived the work and designed the experiments. C.M.T. characterized all the materials and developed the conductive gels, as well as fabricated and characterized all the devices. Y.S., C.C. and K.K. synthesized and provided the graphene material. C.M.T. and H.W. developed and synthesized the PEVM. C.M.T. and A.E.-A. made the material for the alginate gels for the in vitro studies. C.M.T. and I.d.L. carried out the cardiac in vivo recordings, and C.M.T., I.d.L. and B.R.S. conducted the muscular stimulation experiments. F.F. supplied the connectors, F.F. and A.T. performed the neural in vivo recordings, and A.T. analysed the electrocorticography data. C.M.T. and N.V. conducted all the data analysis. C.M.T. and D.J.M. wrote the manuscript, and C.M.T., D.J.M. and S.P.L. discussed the results. All authors contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.M.T. and D.J.M. are inventors on a patent submitted over the viscoelastic conductors and viscoelastic arrays described in this work. C.C. is an inventor on US patent 10421875B2, which covers the graphene materials used in these studies. All the remaining authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Rylie Green, Bozhi Tian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–29, Material 1 and Tables 1 and 2.
Supplementary Video 1
Device stimulating the mouse hindlimb, with two toes responding.
Supplementary Video 2
Device stimulating the mouse hindlimb, with foot responding.
Rights and permissions
About this article
Cite this article
Tringides, C.M., Vachicouras, N., de Lázaro, I. et al. Viscoelastic surface electrode arrays to interface with viscoelastic tissues. Nat. Nanotechnol. 16, 1019–1029 (2021). https://doi.org/10.1038/s41565-021-00926-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-00926-z
This article is cited by
-
3D spatiotemporally scalable in vivo neural probes based on fluorinated elastomers
Nature Nanotechnology (2024)
-
Motion artefact management for soft bioelectronics
Nature Reviews Bioengineering (2024)
-
Network of cyano-p-aramid nanofibres creates ultrastiff and water-rich hydrospongels
Nature Materials (2024)
-
Monolithic-to-focal evolving biointerfaces in tissue regeneration and bioelectronics
Nature Chemical Engineering (2024)
-
Nanoporous graphene-based thin-film microelectrodes for in vivo high-resolution neural recording and stimulation
Nature Nanotechnology (2024)