Abstract
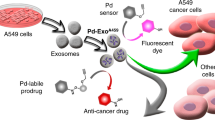
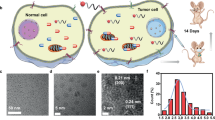
Bioorthogonal catalysis mediated by transition metals has inspired a new subfield of artificial chemistry complementary to enzymatic reactions, enabling the selective labelling of biomolecules or in situ synthesis of bioactive agents via non-natural processes. However, the effective deployment of bioorthogonal catalysis in vivo remains challenging, mired by the safety concerns of metal toxicity or complicated procedures to administer catalysts. Here, we describe a bioorthogonal catalytic device comprising a microneedle array patch integrated with Pd nanoparticles deposited on TiO2 nanosheets. This device is robust and removable, and can mediate the local conversion of caged substrates into their active states in high-level living systems. In particular, we show that such a patch can promote the activation of a prodrug at subcutaneous tumour sites, restoring its parent drug’s therapeutic anticancer properties. This in situ applied device potentiates local treatment efficacy and eliminates off-target prodrug activation and dose-dependent side effects in healthy organs or distant tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files) or are available from the authors upon request.
References
Li, J. & Chen, P. R. Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat. Chem. Biol. 12, 129–137 (2016).
Bai, Y., Chen, J. & Zimmerman, S. C. Designed transition metal catalysts for intracellular organic synthesis. Chem. Soc. Rev. 47, 1811–1821 (2018).
Li, J. et al. Palladium-triggered deprotection chemistry for protein activation in living cells. Nat. Chem. 6, 352–361 (2014).
Tonga, G. Y. et al. Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts. Nat. Chem. 7, 597–603 (2015).
Jeschek, M. et al. Directed evolution of artificial metalloenzymes for in vivo metathesis. Nature 537, 661–665 (2016).
Sancho-Albero, M. et al. Cancer-derived exosomes loaded with ultrathin palladium nanosheets for targeted bioorthogonal catalysis. Nat. Catal. 2, 864–872 (2019).
Eda, S. et al. Biocompatibility and therapeutic potential of glycosylated albumin artificial metalloenzymes. Nat. Catal. 2, 780–792 (2019).
Coverdale, J. P. C. et al. Asymmetric transfer hydrogenation by synthetic catalysts in cancer cells. Nat. Chem. 10, 347–354 (2018).
Vidal, C., Tomás-Gamasa, M., Destito, P., López, F. & Mascareñas, J. L. Concurrent and orthogonal gold(i) and ruthenium(ii) catalysis inside living cells. Nat. Commun. 9, 1913 (2018).
Tomás-Gamasa, M., Martínez-Calvo, M., Couceiro, J. R. & Mascareñas, J. L. Transition metal catalysis in the mitochondria of living cells. Nat. Commun. 7, 12538 (2016).
Wang, X. et al. Copper-triggered bioorthogonal cleavage reactions for reversible protein and cell surface modifications. J. Am. Chem. Soc. 141, 17133–17141 (2019).
Liu, Y. et al. Catalytically active single-chain polymeric nanoparticles: exploring their functions in complex biological media. J. Am. Chem. Soc. 140, 3423–3433 (2018).
Streu, C. & Meggers, E. Ruthenium-induced allylcarbamate cleavage in living cells. Angew. Chem. Int. Ed. 45, 5645–5648 (2006).
Spicer, C. D., Triemer, T. & Davis, B. G. Palladium-mediated cell-surface labeling. J. Am. Chem. Soc. 134, 800–803 (2012).
Li, J. et al. Ligand-free palladium-mediated site-specific protein labeling inside gram-negative bacterial pathogens. J. Am. Chem. Soc. 135, 7330–7338 (2013).
Völker, T., Dempwolff, F., Graumann, P. L. & Meggers, E. Progress towards bioorthogonal catalysis with organometallic compounds. Angew. Chem. Int. Ed. 53, 10536–10540 (2014).
Tsubokura, K. et al. In vivo gold complex catalysis within live mice. Angew. Chem. Int. Ed. 56, 3579–3584 (2017).
Yusop, R. M., Unciti-Broceta, A., Johansson, E. M. V., Sánchez-Martín, R. M. & Bradley, M. Palladium-mediated intracellular chemistry. Nat. Chem. 3, 239–243 (2011).
Wang, F., Zhang, Y., Du, Z., Ren, J. & Qu, X. Designed heterogeneous palladium catalysts for reversible light-controlled bioorthogonal catalysis in living cells. Nat. Commun. 9, 1209 (2018).
Weiss, J. T. et al. Extracellular palladium-catalysed dealkylation of 5-fluoro-1-propargyl-uracil as a bioorthogonally activated prodrug approach. Nat. Commun. 5, 3277 (2014).
Pérez-López, A. M. et al. Gold-triggered uncaging chemistry in living systems. Angew. Chem. Int. Ed. 56, 12548–12552 (2017).
Clavadetscher, J. et al. Copper catalysis in living systems and in situ drug synthesis. Angew. Chem. Int. Ed. 55, 15662–15666 (2016).
Bray, T. L. et al. Bright insights into palladium-triggered local chemotherapy. Chem. Sci. 9, 7354–7361 (2018).
Wang, F. et al. A biocompatible heterogeneous MOF–Cu catalyst for in vivo drug synthesis in targeted subcellular organelles. Angew. Chem. Int. Ed. 58, 6987–6992 (2019).
Miller, M. A. et al. Nano-palladium is a cellular catalyst for in vivo chemistry. Nat. Commun. 8, 15906 (2017).
Prausnitz, M. R. & Langer, R. Transdermal drug delivery. Nat. Biotechnol. 26, 1261–1268 (2008).
Lee, K. et al. Non-transdermal microneedles for advanced drug delivery. Adv. Drug Deliv. Rev. 165-166, 41–59 (2020).
Chen, Z., Hu, Q. & Gu, Z. Leveraging engineering of cells for drug delivery. Acc. Chem. Res. 51, 668–677 (2018).
Li, W. et al. Rapidly separable microneedle patch for the sustained release of a contraceptive. Nat. Biomed. Eng. 3, 220–229 (2019).
Chen, G. et al. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc. Natl Acad. Sci. USA 117, 3687–3692 (2020).
Yang, S. Y. et al. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 4, 1702 (2013).
Yu, J. et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl Acad. Sci. USA 112, 8260–8265 (2015).
Yu, J. et al. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 4, 499–506 (2020).
Wang, Z. et al. Dual self-regulated delivery of insulin and glucagon by a hybrid patch. Proc. Natl Acad. Sci. USA 117, 29512–29517 (2020).
Ye, Y. et al. A melanin-mediated cancer immunotherapy patch. Sci. Immunol. 2, eaan5692 (2017).
Sullivan, S. P. et al. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 16, 915–920 (2010).
DeMuth, P. C., Garcia-Beltran, W. F., Ai-Ling, M. L., Hammond, P. T. & Irvine, D. J. Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv. Funct. Mater. 23, 161–172 (2013).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Fasolino, A., Los, J. H. & Katsnelson, M. I. Intrinsic ripples in graphene. Nat. Mater. 6, 858–861 (2007).
Hassan, C. M. & Peppas, N. A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 33, 2472–2479 (2000).
Yang, S. et al. Phase-transition microneedle patches for efficient and accurate transdermal delivery of insulin. Adv. Funct. Mater. 25, 4633–4641 (2015).
Samant, P. P. & Prausnitz, M. R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl Acad. Sci. USA 115, 4583–4588 (2018).
Rautio, J., Meanwell, N. A., Di, L. & Hageman, M. J. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 17, 559–587 (2018).
Nitiss, J. L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 9, 338–350 (2009).
Qu, X. & Chaires, J. B. in Methods in Enzymology Vol. 321 353–369 (Academic Press, 2000).
Melber, C., Keller, D. & Mangelsdorf, I. Environmental Health Criteria 226: Palladium (World Health Organization, 2002).
Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 16071 (2016).
Li, Y., Boone, E. & El-Sayed, M. A. Size effects of PVP−Pd nanoparticles on the catalytic Suzuki reactions in aqueous solution. Langmuir 18, 4921–4925 (2002).
Acknowledgements
This work was supported by grants from the start-up packages of the University of North Carolina and North Carolina State University (Z.G.), the University of California at Los Angeles (Z.G.), Zhejiang University (Z.G.) and Fuzhou University (0041-510889, Z.C.); a Sloan Research Fellowship of the Alfred P. Sloan Foundation (Z.G.); and the Jonsson Comprehensive Cancer Center at the University of California at Los Angeles (Z.G.). Part of this work was performed at the Analytical Instrumentation Facility at North Carolina State University, which is supported by the state of North Carolina and the National Science Foundation (grant no. 1542015). The Analytical Instrumentation Facility is a member of the North Carolina Research Triangle Nanotechnology Network, a site in the National Nanotechnology Coordinated Infrastructure. We thank L. Huang from the University of North Carolina at Chapel Hill and Z. Li from Hebei University for providing bioluminescent B16-F10 cells and B16-RFP cells, respectively. The XANES and EXAFS studies of this work used resources of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy Office of Science by Argonne National Laboratory, and was supported by the US Department of Energy under contract no. DE-AC02-06CH11357 and by the Canadian Light Source and its funding partners.
Author information
Authors and Affiliations
Contributions
Z.C. and Z.G. designed the project. Z.C., H.L., Y.B., Z.W., G.C., X.Z., Y.M., D.W., J.W., G.W., C.-J.S., J.F. and Y.Z. performed the experiments. Z.C., H.L., Y.B., G.C., Y.M., D.W., J.W., G.W. and Z.G. analysed the data. Z.C., H.L., Y.B., Z.W., Y.M., P.A., S.L. and Z.G. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
Z.G. and Z.C. have applied for patents related to this study. Z.G. is a scientific cofounder of ZenCapsule and ZCapsule. The other authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–42.
Rights and permissions
About this article
Cite this article
Chen, Z., Li, H., Bian, Y. et al. Bioorthogonal catalytic patch. Nat. Nanotechnol. 16, 933–941 (2021). https://doi.org/10.1038/s41565-021-00910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-00910-7
This article is cited by
-
Dipeptide coacervates as artificial membraneless organelles for bioorthogonal catalysis
Nature Communications (2024)
-
Remodel the perifollicular microenvironment via Minoxidil-loaded microneedle patch and cold atmospheric plasma for treating androgenetic alopecia
Nano Research (2024)
-
NIR light-activatable dissolving microneedle system for melanoma ablation enabled by a combination of ROS-responsive chemotherapy and phototherapy
Journal of Nanobiotechnology (2023)
-
Scarless wound healing programmed by core-shell microneedles
Nature Communications (2023)
-
Microneedle biomedical devices
Nature Reviews Bioengineering (2023)