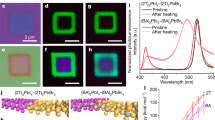
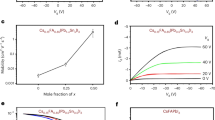
Abstract
Anionic diffusion in a soft crystal lattice of hybrid halide perovskites affects their stability, optoelectronic properties and the resulting device performance. The use of two-dimensional (2D) halide perovskites improves the chemical stability of perovskites and suppresses the intrinsic anionic diffusion in solid-state devices. Based on this strategy, devices with an enhanced stability and reduced hysteresis have been achieved. However, a fundamental understanding of the role of organic cations in inhibiting anionic diffusion across the perovskite–ligand interface is missing. Here we demonstrate the first quantitative investigation of the anionic interdiffusion across atomically flat 2D vertical heterojunctions. Interestingly, the halide diffusion does not follow the classical diffusion process. Instead, a ‘quantized’ layer-by-layer diffusion model is proposed to describe the behaviour of the anionic migration in 2D halide perovskites. Our results provide important insights into the mechanism of anionic diffusion in 2D perovskites and provide a new materials platform with an enhanced stability for heterostructure integration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data are available in the article or its Supplementary Information. All the materials are available upon request to L.D.
References
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Burschka, J. et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499, 316–319 (2013).
Zhou, H. et al. Interface engineering of highly efficient perovskite solar cells. Science 345, 542–546 (2014).
Cao, Y. et al. Perovskite light-emitting diodes based on spontaneously formed submicrometre-scale structures. Nature 562, 249–253 (2018).
Stranks, S. D. & Snaith, H. J. Metal–halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 10, 391–402 (2015).
Zhu, H. et al. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 14, 636–642 (2015).
Saidaminov, M. I. et al. Planar-integrated single-crystalline perovskite photodetectors. Nat. Commun. 6, 8724 (2015).
Snaith, H. J. et al. Anomalous hysteresis in perovskite solar cells. J. Phys. Chem. Lett. 5, 1511–1515 (2014).
Vashishtha, P. & Halpert, J. E. Field-driven ion migration and color instability in red-emitting mixed halide perovskite nanocrystal light-emitting diodes. Chem. Mater. 29, 5965–5973 (2017).
Yuan, Y. & Huang, J. Ion migration in organometal trihalide perovskite and its impact on photovoltaic efficiency and stability. Acc. Chem. Res. 49, 286–293 (2016).
Xiao, Z. et al. Giant switchable photovoltaic effect in organometal trihalide perovskite devices. Nat. Mater. 14, 193–198 (2015).
Eames, C. et al. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 6, 7497 (2015).
Pan, D. et al. Visualization and studies of ion-diffusion kinetics in cesium lead bromide perovskite nanowires. Nano Lett. 18, 1807–1813 (2018).
Zhang, Y. et al. Quantitative imaging of anion exchange kinetics in halide perovskites. Proc. Natl Acad. Sci. USA 116, 12648–12653 (2019).
Lai, M. et al. Intrinsic anion diffusivity in lead halide perovskites is facilitated by a soft lattice. Proc. Natl Acad. Sci. USA 115, 11929–11934 (2018).
Shewmon, N. T., Yu, H., Constantinou, I., Klump, E. & So, F. Formation of perovskite heterostructures by ion exchange. ACS Appl. Mater. Interfaces 8, 33273–33279 (2016).
Saparov, B. & Mitzi, D. B. Organic–inorganic perovskites: structural versatility for functional materials design. Chem. Rev. 116, 4558–4596 (2016).
Gao, Y. et al. Molecular engineering of organic–inorganic hybrid perovskites quantum wells. Nat. Chem. 11, 1151–1157 (2019).
Lin, Y. et al. Suppressed ion migration in low-dimensional perovskites. ACS Energy Lett. 2, 1571–1572 (2017).
Cao, D. H., Stoumpos, C. C., Farha, O. K., Hupp, J. T. & Kanatzidis, M. G. 2D homologous perovskites as light-absorbing materials for solar cell applications. J. Am. Chem. Soc. 137, 7843–7850 (2015).
Smith, I. C., Hoke, E. T., Solis-Ibarra, D., McGehee, M. D. & Karunadasa, H. I. A layered hybrid perovskite solar cell absorber with enhanced moisture stability. Angew. Chem. Int. Ed. 53, 11232–11235 (2014).
Saidaminov, M. I., Mohammed, O. F. & Bakr, O. M. Low-dimensional-networked metal halide perovskites: the next big thing. ACS Energy Lett. 2, 889–896 (2017).
Dou, L. et al. Atomically thin two-dimensional organic–inorganic hybrid perovskites. Science 349, 1518–1521 (2015).
Shi, E. et al. Two-dimensional halide perovskite lateral epitaxial heterostructures. Nature 580, 614–620 (2020).
Wang, J. et al. Controllable synthesis of two-dimensional Ruddlesden–Popper-type perovskite heterostructures. J. Phys. Chem. Lett. 8, 6211–6219 (2017).
Wang, J. X. et al. Controllable growth of centimeter-sized 2D perovskite heterostructures for highly narrow dual-band photodetectors. ACS Nano 13, 5473–5484 (2019).
Hwang, B. H. & Lee, J.-S. 2D perovskite‐based self‐aligned lateral heterostructure photodetectors utilizing vapor deposition. Adv. Opt. Mater. 7, 1801356 (2019).
Fu, Y. et al. Multicolor heterostructures of two-dimensional layered halide perovskites that show interlayer energy transfer. J. Am. Chem. Soc. 140, 15675–15683 (2018).
Yuan, M. et al. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 11, 872–877 (2016).
Wang, N. et al. Perovskite light-emitting diodes based on solution-processed self-organized multiple quantum wells. Nat. Photon. 10, 699–704 (2016).
Xing, G. et al. Transcending the slow bimolecular recombination in lead–halide perovskites for electroluminescence. Nat. Commun. 8, 14558 (2017).
Quan, L. N. et al. Tailoring the energy landscape in quasi-2D halide perovskites enables efficient green-light emission. Nano Lett. 17, 3701–3709 (2017).
Shang, Q. et al. Unveiling structurally engineered carrier dynamics in hybrid quasi-two-dimensional perovskite thin films toward controllable emission. J. Phys. Chem. Lett. 8, 4431–4438 (2017).
Slotcavage, D. J., Karunadasa, H. I. & McGehee, M. D. Light-induced phase segregation in halide–perovskite absorbers. ACS Energy Lett. 1, 1199–1205 (2016).
Brivio, F., Caetano, C. & Walsh, A. Thermodynamic origin of photoinstability in the CH3NH3Pb(I1–xBrx)3 hybrid halide perovskite alloy. J. Phys. Chem. Lett. 7, 1083–1087 (2016).
Yin, W., Yan, Y. & Wei, S. Anomalous alloy properties in mixed halide perovskites. J. Phys. Chem. Lett. 5, 3625–3631 (2014).
Fick, A. Ueber diffusion. Ann. Phys. 170, 59–86 (1855).
Tuck, B. Atomic Diffusion in III–V Semiconductors Ch 2 (IOP, 1988).
Matano, C. On the relation between the diffusion-coefficients and concentrations of solid metals (the nickel–copper system). Jpn J. Phys. 8, 109–113 (1993).
Arrhenius, S. A. Über die dissociationswärme und den einfluß der temperatur auf den dissociationsgrad der elektrolyte. Z. Phys. Chem. 4, 96–116 (1889).
Arrhenius, S. A. Über die reaktionsgeschwindigkeit bei der Inversion von rohrzucker durch säuren. Z. Phys. Chem. 4, 226–248 (1889).
Kresse, G. Ab initio molecular dynamics for liquid metals. J. Non-Cryst. Solids 192-193, 222–229 (1995).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Hafner, G. Ab initio molecular-dynamics simulation of the liquid–metal-amorphous–semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Steiner, S., Khmelevskyi, S., Marsmann, M. & Kresse, G. Calculation of the magnetic anisotropy with projected-augmented-wave methodology and the case study of disordered Fe1–xCox alloys. Phys. Rev. B 93, 224425 (2016).
Stoltze, P. Simulation Methods in Atomic Scale Materials Physics (Polyteknisk, 1977).
Fichthorn, K. A. & Weinberg, W. H. Theoretical foundations of dynamical Monte Carlo simulations. J. Chem. Phys. 95, 1090–1096 (1991).
Acknowledgements
This work is supported by the Office of Naval Research (Grant no N00014-19-1-2296, Program Managers, J. Parker and P. Armistead). Akriti acknowledges support from the Frederick N. Andrews Fellowship. B.P.F. acknowledges support from the Purdue Process Safety & Assurance Center. B.M.S. acknowledges the Air Force Office of Scientific Research (AFOSR) for their support (Grant no. FA9550-18-S-0003, Program Manager, K. Caster). The DFT computational work by P.L. and J.Y. is supported in part through computational resources provided by the Information Technology Department at Purdue University. The transmission electron microscopy work is supported by funding from the National Natural Science Foundation of China (Grant no. 21805184), the Natural Science Foundation of Shanghai (Grant no. 18ZR1425200) and the Center for High-Resolution Electron Microscopy (CћEM) at ShanghaiTech University (Grant no. EM02161943). We thank Q. Zhao, L. Huang, R. Agrawal, B. W. Boudouris and C. Davis for helpful discussions.
Author information
Authors and Affiliations
Contributions
Akriti and E.S. synthesized and characterized the 2D perovskite vertical heterostructures; Akriti performed the analytical calculations of the halide interdiffusion coefficients; S.B.S. and B.M.S. performed the KMC simulations and data analysis; J.Y. and P.L. carried out the DFT calculations; C.L.A.-M. and A.J.P. participated in the heterostructure fabrication and optical characterization; B.Y., X.H. and Y.Y. conducted the STEM characterization; Y.G. performed the organic ligand synthesis; B.P.F. performed the SEM and EDS characterizations; Akriti and L.D. wrote the manuscript; all the authors read and revised the manuscript. L.D. supervised the project.
Corresponding author
Ethics declarations
Competing interests
Authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Dehui Li, Biwu Ma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cross-sectional characterization of (BA)2PbBr4-(BA)2(MA)2Pb3I10 vertical heterostructure.
a, SEM image of the vertical heterostructure (top view). The dashed rectangle highlights the area used for cross-sectional characterization. b, Carbon coating of the rectangular area of interest. Preparation of the cross-sectional lamella by c, digging a hole and d, thinning by Ga ions. e, Prepared cross-section of the vertical heterostructure. Scale bars in a–e are 10 µm. f, Low-magnification STEM image of the vertical heterostructure. The scale bar is 1 µm. g, Enlarged STEM image of the vertical heterostructure. STEM images show a clear interface between (BA)2PbBr4 and (BA)2(MA)2Pb3I10. EDS elemental mapping of h, Pb, i, Br, and j, I. k, Overlaid bromine and iodine EDS mapping. Scale bars in g–k are 200 nm. The brighter bromine and iodine signals in the EDS elemental mappings are mainly concentrated in the pure phase (BA)2PbBr4 and (BA)2(MA)2Pb3I10 regions, respectively. The dimmer Br signal from (BA)2(MA)2Pb3I10 region and iodine signal from (BA)2PbBr4 region can be attributed to diffusion, which is likely caused by the harsh sputtering and focused ion-beam milling during the cross-sectional sample preparation. These results suggest that electron microscopy is not an ideal tool to study the anion inter-diffusion kinetics for 2D halide perovskites due to sample damage.
Extended Data Fig. 2 Analysis of origin of halide alloy PL peak in (BA)2PbBr4-(BA)2(MA)2Pb3I10 vertical heterostructure after heating at 80 °C.
a, Bright-field image of vertical heterostructure. b, c, PL image of vertical heterostructure at room temperature and after heating at 80 °C for 40 hrs, respectively. The circled regions denote the area used for PL emission measurement of the heterostructure, bromide perovskite and iodide perovskite. Dashed lines are added in all the bright-field and PL images for the ease of distinction of bromide (light blue) and iodide (yellow) domains. Scale bars in a–c are 10 µm. d, PL spectrum of the vertical heterostructure before and after heating at 80 °C for 40 hrs. Presence of halide alloy PL peak at ~ 511 nm (dashed blue curve) is evident in bromide perovskite layer. No new PL peak (dashed red curve) is observed in iodide perovskite layer after the heating process. e, f, Bright-field and PL image of (BA)2PbBr4, respectively. g, PL image of the (BA)2PbBr4xI4(1−x) alloy formed after placing bulk crystal of (BA)2(MA)2Pb3I10 on top of (BA)2PbBr4 nanocrystal and heating it for 4 hrs at 80 °C. The bulk crystal of (BA)2(MA)2Pb3I10 is removed after the heating process to analyze the formation of (BA)2PbBr4xI4(1-x) perovskite in the bottom layer. Scale bars in e–g are 5 µm. h, PL spectrum of (BA)2PbBr4 nanocrystal at room temperature and (BA)2PbBr4xI4(1-x) alloy formed in the bottom layer after heating (BA)2PbBr4-(BA)2(MA)2Pb3I10 vertical heterostructure at 80 °C for 4 hrs.
Extended Data Fig. 3 SEM / EDS imaging of (BA)2PbBr4-(BA)2(MA)2Pb3I10 vertical heterostructure.
SEM images of (BA)2PbBr4xI4(1-x) alloy formed after placing bulk crystal of (BA)2(MA)2Pb3I10 on top of (BA)2PbBr4 nanocrystal and heating it for a, 4 hrs and e, 10 hrs at 80 °C. EDS elemental mappings of b, f, Pb, c, g, Br, and d, h, I for the SEM images in a and e, respectively. All scale bars are 3 µm. i, j, EDS spectrum corresponding to the SEM images in a and e, respectively. The insets show the zoomed-in spectrum to highlight the bromine and iodine counts. The weak iodine signal in EDS elemental mapping and spectrum after 4 hrs of heating suggest that the iodine ions diffused to only top few layers of the bromide perovskite after 4 hrs of heating. After 10 hrs of heating, almost all the bromide perovskite layers show dominant iodine composition as evident from the EDS mapping and spectrum.
Extended Data Fig. 4 Analysis of new phase formation in (BA)2PbX4-(BA)2(MA)2Pb3X10 (X = I, Br) and alloy formation in (BA)2(MA)2Pb3Br10-(BA)2PbI4 vertical heterostructures after heating at 80 °C.
a, Bright-field image of (BA)2PbI4-(BA)2(MA)2Pb3I10 vertical heterostructure. b, c, PL images of (BA)2PbI4-(BA)2(MA)2Pb3I10 vertical heterostructure at room temperature and after heating at 80 °C for 4 hrs, respectively. d, PL spectrum of (BA)2PbI4-(BA)2(MA)2Pb3I10 vertical heterostructure before and after heating at 80 °C for 4 hrs. No PL shift and no new PL emission from any new halide perovskite phases is observed after the heating process. e, Bright-field image of (BA)2PbBr4-(BA)2(MA)2Pb3Br10 vertical heterostructure. f, g, PL images of (BA)2PbBr4-(BA)2(MA)2Pb3Br10 vertical heterostructure at room temperature and after heating at 80 °C for 4 hrs, respectively. h, PL spectrum of (BA)2PbBr4-(BA)2(MA)2Pb3Br10 vertical heterostructure before and after heating at 80 °C for 4 hrs. No PL shift and no new PL emission from any new halide perovskite phases is observed after the heating process. i, Bright-field image of (BA)2(MA)2Pb3Br10-(BA)2PbI4 vertical heterostructure. j, k, PL images of (BA)2(MA)2Pb3Br10-(BA)2PbI4 vertical heterostructure at room temperature and after heating at 80 °C for 4 hrs, respectively. l, PL spectrum of (BA)2(MA)2Pb3Br10-(BA)2PbI4 vertical heterostructure before and after heating at 80 °C. Alloy peak centered ~ 605 nm observed after heating indicates formation of iodide dominant (BA)2(MA)2Pb3Br10xI10(1-x) (n = 3) alloy perovskite rather than (BA)2PbBr4xI4(1-x) (n = 1) alloy perovskite. Dashed lines are added in all the bright-field and PL images for the ease of distinction of bottom (light blue) and top (yellow) perovskite domains. All scale bars are 5 µm.
Extended Data Fig. 5 Investigation of halide inter-diffusion in (2T)2PbBr4-(BA)2(MA)2Pb3I10 vertical heterostructure.
a, SEM image of bottom bromide perovskite layer after placing bulk crystal of (BA)2(MA)2Pb3I10 on top of (2 T)2PbBr4 nanocrystal and heating it for 10 hrs at 80 °C. b, c, d, EDS elemental mappings of Pb, Br, and I, respectively, for the SEM image in a. Scale bars in a–d are 10 µm. e, EDS spectrum corresponding to the SEM image in a. f, g, h, i, j, PL images of thin films of (2T)2PbBr4xI4(1-x) alloy perovskite with xBr = 0.4, 0.3, 0.2, 0.1, and 0, respectively. All scale bars are 20 µm. k, PL emission spectrum of alloy perovskites with xBr = 1,0.9, 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, 0.2, 0.1, and 0. The legend texts for xBr > 0.4 have been shown in grey color to highlight that no PL is observed for these compositions. Note that (2T)2PbBr4 perovskite has no PL emission due to type II band alignment between the inorganic [PbBr4]2− and organic ligand 2T+ layers. The red PL emission in (2T)2PbBr4-(BA)2(MA)2Pb3I10 vertical heterostructure comes from the top iodide perovskite layer. However, (2T)2PbI4 has type I band alignment with green PL emission. As all other heterostructures show an alloy PL emission peak which is iodide dominant, we expected the halide inter-diffusion in 2T system to be thermodynamically driven towards formation of an iodine dominated (2T)2PbBr4xI4(1-x) perovskite with green PL emission. To ensure that absence of green alloy PL emission in 2T system indicates absence of iodide diffusion rather than quenched PL of iodide dominant alloy perovskite, it is important to estimate the bromide concentration at which the (2T)2PbBr4xI4(1-x) alloy perovskite transitions from type II to type I band alignment. Extended Data Fig. 5f–k shows the PL emission spectrum of (2T)2PbBr4xI4(1-x) alloy perovskites obtained by spin coating the bromide and iodide precursor solutions mixed in the desired stoichiometric ratios. It is evident from Extended Data Fig. 5k that for xBr ≤ 0.4, (2T)2PbBr4xI4(1-x) perovskite has type I band alignment and for xBr > 0.4, it transitions to type II band alignment. Therefore, the absence of alloy PL emission in the diffusion studies is indicative of the inhibiting role of 2T organic cation rather than type II band alignment of iodide dominant alloy perovskites.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16 and Table 1.
Rights and permissions
About this article
Cite this article
Akriti, Shi, E., Shiring, S.B. et al. Layer-by-layer anionic diffusion in two-dimensional halide perovskite vertical heterostructures. Nat. Nanotechnol. 16, 584–591 (2021). https://doi.org/10.1038/s41565-021-00848-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-00848-w
This article is cited by
-
Two-dimensional lead halide perovskite lateral homojunctions enabled by phase pinning
Nature Communications (2024)
-
A multiscale ion diffusion framework sheds light on the diffusion–stability–hysteresis nexus in metal halide perovskites
Nature Materials (2023)
-
Enabling low-drift flexible perovskite photodetectors by electrical modulation for wearable health monitoring and weak light imaging
Nature Communications (2023)
-
Optical properties of two-dimensional perovskites
Frontiers of Physics (2023)
-
Suppressing phase disproportionation in quasi-2D perovskite light-emitting diodes
Nature Communications (2023)