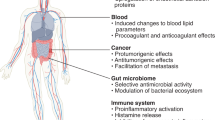
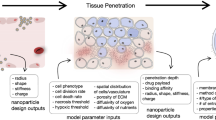
Abstract
The delivery of medical agents to a specific diseased tissue or cell is critical for diagnosing and treating patients. Nanomaterials are promising vehicles to transport agents that include drugs, contrast agents, immunotherapies and gene editors. They can be engineered to have different physical and chemical properties that influence their interactions with their biological environments and delivery destinations. In this Review Article, we discuss nanoparticle delivery systems and how the biology of disease should inform their design. We propose developing a framework for building optimal delivery systems that uses nanoparticle–biological interaction data and computational analyses to guide future nanomaterial designs and delivery strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Walkey, C. D. et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 8, 2439–2455 (2014).
Tenzer, S. et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 8, 772–781 (2013).
Monopoli, M. P. et al. Physical−chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 133, 2525–2534 (2011).
Walczyk, D., Bombelli, F. B., Monopoli, M. P., Lynch, I. & Dawson, K. A. What the cell ‘sees’ in bionanoscience. J. Am. Chem. Soc. 132, 5761–5768 (2010).
Monopoli, M. P., Åberg, C., Salvati, A. & Dawson, K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 7, 779–786 (2012).
Varnamkhasti, B. S. et al. Protein corona hampers targeting potential of MUC1 aptamer functionalized SN-38 core–shell nanoparticles. Int. J. Pharm. 494, 430–444 (2015).
Salvati, A. et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 8, 137–143 (2013).
Schipper, M. L. et al. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small 5, 126–134 (2009).
Fonge, H., Huang, H., Scollard, D., Reilly, R. M. & Allen, C. Influence of formulation variables on the biodistribution of multifunctional block copolymer micelles. J. Control. Release 157, 366–374 (2012).
De Jong, W. H. et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 29, 1912–1919 (2008).
Lee, J. S. et al. Circulation kinetics and biodistribution of dual-labeled polymersomes with modulated surface charge in tumor-bearing mice: comparison with stealth liposomes. J. Control. Release 155, 282–288 (2011).
Shimada, K. et al. Biodistribution of liposomes containing synthetic galactose-terminated diacylglyceryl-poly(ethyleneglycol)s. Biochim. Biophys. Acta 1326, 329–341 (1997).
Sadauskas, E. et al. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine 5, 162–169 (2009).
Tsoi, K. M. et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 15, 1212–1221 (2016).
Liliemark, E. et al. Targeting of teniposide to the mononuclear phagocytic system (MPS) by incorporation in liposomes and submicron lipid particles; an autoradiographic study in mice. Leuk. Lymphoma 18, 113–118 (1995).
Sun, X. et al. Improved tumor uptake by optimizing liposome based RES blockade strategy. Theranostics 7, 319–328 (2017).
Klibanov, A. L., Maruyama, K., Torchilin, V. P. & Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 268, 235–237 (1990).
Choi, H. S. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Kwon, Y. J., James, E., Shastri, N. & Frechet, J. M. J. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc. Natl Acad. Sci. 102, 18264–18268 (2005).
Poon, W. et al. Elimination pathways of nanoparticles. ACS Nano 13, 5785–5798 (2019).
Lawrence, M. G. et al. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc. Natl Acad. Sci. USA 114, 2958–2963 (2017).
Choi, H. S. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Satchell, S. C. & Braet, F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am. J. Physiol. Ren. Physiol. 296, F947–56 (2009).
Satchell, S. C. The glomerular endothelium emerges as a key player in diabetic nephropathy. Kidney Int. 82, 949–951 (2012).
Du, B. et al. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 12, 1096–1102 (2017).
Balogh, L. et al. Significant effect of size on the in vivo biodistribution of gold composite nanodevices in mouse tumor models. Nanomedicine 3, 281–296 (2007).
Du, B., Yu, M. & Zheng, J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 3, 358–374 (2018).
Ruggiero, A. et al. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl Acad. Sci. USA 107, 12369–12374 (2010).
Jasim, D. A. et al. The effects of extensive glomerular filtration of thin graphene oxide sheets on kidney physiology. ACS Nano 10, 10753–10767 (2016).
Saraiva, C. et al. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 235, 34–47 (2016).
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. https://doi.org/10.1038/s41563-019-0566-2 (2020).
Wiley, D. T., Webster, P., Gale, A. & Davis, M. E. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc. Natl Acad. Sci. USA. 110, 8662–8667 (2013).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Karsdal, M. A. et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G807–30 (2015).
Cox, T. R. & Erler, J. T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Model. Mech. 4, 165–178 (2011).
Sykes, E. A. et al. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc. Natl Acad. Sci. USA 113, E1142–E1151 (2016).
Netti, P. A., Berk, D. A., Swartz, M. A., Grodzinsky, A. J. & Jain, R. K. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 60, 2497–2503 (2000).
Dai, Q. et al. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano 12, 8423–8435 (2018).
Miller, M. A. et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 6, 8692 (2015).
Korangath, P. et al. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell-mediated tumor suppression in models of breast cancer. Sci. Adv. 6, eaay1601 (2020).
Miller, M. A. et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 7, 314ra183 (2015).
Cuccarese, M. F. et al. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat. Commun. 8, 14293 (2017).
Kim, H.-Y. et al. Quantitative imaging of tumor-associated macrophages and their response to therapy using 64Cu-Labeled Macrin. ACS Nano 12, 12015–12029 (2018).
Düzgüneş, N. & Nir, S. Mechanisms and kinetics of liposome–cell interactions. Adv. Drug Deliv. Rev. 40, 3–18 (1999).
Sahay, G., Kim, J. O., Kabanov, A. V. & Bronich, T. K. The exploitation of differential endocytic pathways in normal and tumor cells in the selective targeting of nanoparticulate chemotherapeutic agents. Biomaterials 31, 923–933 (2010).
Harush-Frenkel, O., Debotton, N., Benita, S. & Altschuler, Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem. Biophys. Res. Commun. 353, 26–32 (2007).
Meng, H. et al. Aspect ratio determines the quantity of mesoporous silica nanoparticle uptake by a small GTPase-dependent macropinocytosis mechanism. ACS Nano 5, 4434–4447 (2011).
Lunov, O. et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 5, 1657–1669 (2011).
van de Water, B. & van de Water, B. Quantitative assessment of mitochondrial toxicity and downstream cellular perturbations in adverse outcome pathways. Toxicol. Lett. 295, S32 (2018).
dos Santos, T., Varela, J., Lynch, I., Salvati, A. & Dawson, K. A. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS One 6, e24438 (2011).
Hafez, I. M., Maurer, N. & Cullis, P. R. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 8, 1188–1196 (2001).
Akinc, A., Thomas, M., Klibanov, A. M. & Langer, R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 7, 657–663 (2005).
Pack, D. W., Putnam, D. & Langer, R. Design of imidazole-containing endosomolytic biopolymers for gene delivery. Biotechnol. Bioeng. 67, 217–223 (2000).
Hu, Y. et al. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using pH-responsive core−shell nanoparticles. Nano Lett. 7, 3056–3064 (2007).
Pan, L. et al. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 134, 5722–5725 (2012).
Nakielny, S. & Dreyfuss, G. Transport of Proteins and RNAs in and out of the Nucleus. Cell 99, 677–690 (1999).
Garbuzenko, O. B. et al. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc. Natl Acad. Sci. USA 107, 10737–10742 (2010).
Griffin, J. I. et al. Revealing dynamics of accumulation of systemically injected liposomes in the skin by intravital microscopy. ACS Nano 11, 11584–11593 (2017).
Moghimi, S. M., Hunter, A. C. & Murray, J. C. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 53, 283–318 (2001).
Lotem, M. et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch. Dermatol. 136, 1475–1480 (2000).
Lu, M., Cohen, M. H., Rieves, D. & Pazdur, R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am. J. Hematol. 85, 315–319 (2010).
Lu, F., Wu, S.-H., Hung, Y. & Mou, C.-Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 5, 1408–1413 (2009).
Jin, H., Heller, D. A., Sharma, R. & Strano, M. S. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. ACS Nano 3, 149–158 (2009).
Agarwal, R. et al. Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc. Natl Acad. Sci. USA 110, 17247–17252 (2013).
Huang, X., Teng, X., Chen, D., Tang, F. & He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 31, 438–448 (2010).
Wang, Z., Zhang, J., Ekman, J. M., Kenis, P. J. A. & Lu, Y. DNA-mediated control of metal nanoparticle shape: one-pot synthesis and cellular uptake of highly stable and functional gold nanoflowers. Nano Lett. 10, 1886–1891 (2010).
Elias, D. R., Poloukhtine, A., Popik, V. & Tsourkas, A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine 9, 194–201 (2013).
Giljohann, D. A. et al. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 7, 3818–3821 (2007).
Bai, X. et al. Regulation of cell uptake and cytotoxicity by nanoparticle core under the controlled shape, size, and surface chemistries. ACS Nano 14, 289–302 (2020).
Clift, M. J. D. et al. The impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell line. Toxicol. Appl. Pharmacol. 232, 418–427 (2008).
Oh, N. & Park, J.-H. Surface chemistry of gold nanoparticles mediates their exocytosis in macrophages. ACS Nano 8, 6232–6241 (2014).
Wang, J., Min, J., Eghtesadi, S. A., Kane, R. S. & Chilkoti, A. Quantitative study of the interaction of multivalent ligand-modified nanoparticles with breast cancer cells with tunable receptor density. ACS Nano 14, 372–383 (2020).
Ekdawi, S. N. et al. Spatial and temporal mapping of heterogeneity in liposome uptake and microvascular distribution in an orthotopic tumor xenograft model. J. Control. Release 207, 101–111 (2015).
Kingston, B. R., Syed, A. M., Ngai, J., Sindhwani, S. & Chan, W. C. W. Assessing micrometastases as a target for nanoparticles using 3D microscopy and machine learning. Proc. Natl Acad. Sci. USA 116, 14937–14946 (2019).
Stirland, D. L., Matsumoto, Y., Toh, K., Kataoka, K. & Bae, Y. H. Analyzing spatiotemporal distribution of uniquely fluorescent nanoparticles in xenograft tumors. J. Control. Release 227, 38–44 (2016).
Kai, M. P. et al. Tumor Presence Induces Global Immune Changes and Enhances Nanoparticle Clearance. ACS Nano 10, 861–870 (2016).
Wu, H. et al. Population pharmacokinetics of pegylated liposomal CKD-602 (S-CKD602) in patients with advanced malignancies. J. Clin. Pharmacol. 52, 180–194 (2012).
Lazarovits, J. et al. Supervised learning and mass spectrometry predicts the fate of nanomaterials. ACS Nano 13, 8023–8034 (2019).
Liu, R., Jiang, W., Walkey, C. D., Chan, W. C. W. & Cohen, Y. Prediction of nanoparticles-cell association based on corona proteins and physicochemical properties. Nanoscale 7, 9664–9675 (2015).
Ban, Z. et al. Machine learning predicts the functional composition of the protein corona and the cellular recognition of nanoparticles. Proc. Natl Acad. Sci. USA 117, 10492–10499 (2020).
Fourches, D. et al. Quantitative nanostructure−activity relationship modeling. ACS Nano 4, 5703–5712 (2010).
Puzyn, T. et al. Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nat. Nanotechnol. 6, 175–178 (2011).
Paunovska, K., Loughrey, D., Sago, C. D., Langer, R. & Dahlman, J. E. Using Large Datasets to Understand Nanotechnology. Adv. Mater. 31, e1902798 (2019).
Yamankurt, G. et al. Exploration of the nanomedicine-design space with high-throughput screening and machine learning. Nat. Biomed. Eng. 3, 318–327 (2019).
Ng, T. S. C., Garlin, M. A., Weissleder, R. & Miller, M. A. Improving nanotherapy delivery and action through image-guided systems pharmacology. Theranostics 10, 968–997 (2020).
Lee, H. et al. Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 23, 4190–4202 (2017).
Syed, A. M. et al. Liposome imaging in optically cleared tissues. Nano Lett. 20, 1362–1369 (2020).
Koo, D.-J. et al. Large-scale 3D optical mapping and quantitative analysis of nanoparticle distribution in tumor vascular microenvironment. Bioconjug. Chem. https://doi.org/10.1021/acs.bioconjchem.0c00263 (2020).
Tavares, A. J. et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc. Natl Acad. Sci. USA 114, E10871–E10880 (2017).
Souhami, R. L., Patel, H. M. & Ryman, B. E. The effect of reticuloendothelial blockade on the blood clearance and tissue distribution of liposomes. Biochim. Biophys. Acta 674, 354–371 (1981).
Liu, D., Mori, A. & Huang, L. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim. Biophys. Acta 1104, 95–101 (1992).
Proffitt, R. T. et al. Liposomal blockade of the reticuloendothelial system: improved tumor imaging with small unilamellar vesicles. Science 220, 502–505 (1983).
Ouyang, B. et al. The dose threshold for nanoparticle tumour delivery. Nat. Mater. https://doi.org/10.1038/s41563-020-0755-z (2020).
Chauhan, V. P. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 7, 383–388 (2012).
Arjaans, M. et al. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res. 73, 3347–3355 (2013).
Chen, Y. et al. Therapeutic remodeling of the tumor microenvironment enhances nanoparticle delivery. Adv. Sci. 6, 1802070 (2019).
Miller, M. A. et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci. Transl. Med. 9, eaal0225 (2017).
Kunjachan, S. et al. Selective priming of tumor blood vessels by radiation therapy enhances nanodrug delivery. Sci. Rep. 9, 15844 (2019).
Herrera, J., Henke, C. A. & Bitterman, P. B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Invest. 128, 45–53 (2018).
McKee, T. D. et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 66, 2509–2513 (2006).
Gong, H. et al. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 16, 2512–2521 (2016).
Li, X. et al. Parallel accumulation of tumor hyaluronan, collagen, and other drivers of tumor progression. Clin. Cancer Res. 24, 4798–4807 (2018).
Murty, S. et al. Nanoparticles functionalized with collagenase exhibit improved tumor accumulation in a murine xenograft model. Part. Part. Syst. Charact. 31, 1307–1312 (2014).
Eikenes, L., Tari, M., Tufto, I., Bruland, Ø. S. & de Lange Davies, C. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (CaelyxTM) in human osteosarcoma xenografts. Br. J. Cancer 93, 81–88 (2005).
Enriquez-Navas, P. M. et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci. Transl. Med. 8, 327ra24 (2016).
Zarrinpar, A. et al. Individualizing liver transplant immunosuppression using a phenotypic personalized medicine platform. Sci. Transl. Med. 8, 333ra49 (2016).
Pantuck, A. J. et al. Modulating BET bromodomain inhibitor ZEN-3694 and enzalutamide combination dosing in a metastatic prostate cancer patient using CURATE.AI, an artificial intelligence platform. Adv. Ther. 1, 1800104 (2018).
Lou, B. et al. An image-based deep learning framework for individualising radiotherapy dose: a retrospective analysis of outcome prediction. Lancet Digit. Health 1, e136–e147 (2019).
Tang, J. et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl Acad. Sci. USA 113, E6731–E6740 (2016).
Dahlman, J. E. et al. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc. Natl Acad. Sci. USA 114, 2060–2065 (2017).
Sago, C. D. et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc. Natl Acad. Sci. USA 115, E9944–E9952 (2018).
Mu, Q. et al. Conjugate-SELEX: A high-throughput screening of thioaptamer-liposomal nanoparticle conjugates for targeted intracellular delivery of anticancer drugs. Mol. Ther. Nucleic Acids 5, e382 (2016).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25, 44–56 (2019).
Chamunyonga, C., Edwards, C., Caldwell, P., Rutledge, P. & Burbery, J. The impact of artificial intelligence and machine learning in radiation therapy: Considerations for future curriculum enhancement. J. Med. Imaging Radiat. Sci. 51, 214–220 (2020).
McNeil, S. E. Evaluation of nanomedicines: stick to the basics. Nat. Rev. Mater. 1, 16073 (2016).
Acknowledgements
W.C.W.C. acknowledges the Canadian Institute of Health Research (CIHR, FDN-159932; MOP-130143), Natural Sciences and Engineering Research Council of Canada (NSERC, 2015–06397), Canadian Research Chairs program (950–223824), Collaborative Health Research Program (CPG-146468) and Canadian Cancer Society (705185–1) for funding support. We also acknowledge CIHR (W.P., B.O.), Vanier Canada Graduate Scholarships (B.O.), Ontario Graduate Scholarship (W.P., B.O.), NSERC (B.R.K., W.N.), Barbara and Frank Milligan (W. P.), Wildcat Foundation (B.R.K., W.N.), Jennifer Dorrington Award (B.R.K.), Royal Bank of Canada and Borealis AI (B.R.K.), Frank Fletcher Memorial Fund (B.O.), John J. Ruffo (B.O.), Cecil Yip family (W.P., B.R.K., B.O., W.N.) and McLaughlin Centre for MD/PhD studentships (B.O.) for financial support. The authors thank S. Sindhwani, J. Ngai, J. L. Y. Wu, and Z. Sepahi for manuscript revisions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Poon, W., Kingston, B.R., Ouyang, B. et al. A framework for designing delivery systems. Nat. Nanotechnol. 15, 819–829 (2020). https://doi.org/10.1038/s41565-020-0759-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0759-5
This article is cited by
-
The remodeling of ovarian function: targeted delivery strategies for mesenchymal stem cells and their derived extracellular vesicles
Stem Cell Research & Therapy (2024)
-
Physiological principles underlying the kidney targeting of renal nanomedicines
Nature Reviews Nephrology (2024)
-
Strategies for non-viral vectors targeting organs beyond the liver
Nature Nanotechnology (2024)
-
Intracerebral fate of organic and inorganic nanoparticles is dependent on microglial extracellular vesicle function
Nature Nanotechnology (2024)
-
In situ label-free X-ray imaging for visualizing the localization of nanomedicines and subcellular architecture in intact single cells
Nature Protocols (2024)