Abstract
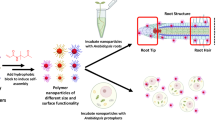
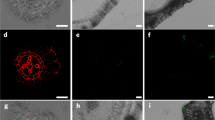
Although the fates of microplastics (0.1–5 mm in size) and nanoplastics (<100 nm) in marine environments are being increasingly well studied1,2, little is known about the behaviour of nanoplastics in terrestrial environments3,4,5,6, especially agricultural soils7. Previous studies have evaluated the consequences of nanoplastic accumulation in aquatic plants, but there is no direct evidence for the internalization of nanoplastics in terrestrial plants. Here, we show that both positively and negatively charged nanoplastics can accumulate in Arabidopsis thaliana. The aggregation promoted by the growth medium and root exudates limited the uptake of amino-modified polystyrene nanoplastics with positive surface charges. Thus, positively charged nanoplastics accumulated at relatively low levels in the root tips, but these nanoplastics induced a higher accumulation of reactive oxygen species and inhibited plant growth and seedling development more strongly than negatively charged sulfonic-acid-modified nanoplastics. By contrast, the negatively charged nanoplastics were observed frequently in the apoplast and xylem. Our findings provide direct evidence that nanoplastics can accumulate in plants, depending on their surface charge. Plant accumulation of nanoplastics can have both direct ecological effects and implications for agricultural sustainability and food safety.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The processed RNA-Seq data have been deposited in the Gene Expression Omnibus database under accession code GSE123369. All the other data are available from the corresponding author upon reasonable request.
References
Galloway, T. S., Cole, M. & Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 1, 0116 (2017).
Dawson, A. L. et al. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 9, 1001 (2018).
Weithmann, N. et al. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 4, 8060 (2018).
Huerta Lwanga, E. et al. Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 50, 2685–2691 (2016).
Hodson, M. E., Duffus-Hodson, C. A., Clark, A., Prendergast-Miller, M. T. & Thorpe, K. L. Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ. Sci. Technol. 51, 4714–4721 (2017).
de Souza Machado, A. A., Kloas, W., Zarfl, C., Hempel, S. & Rillig, M. C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 24, 1405–1416 (2018).
Nizzetto, L., Langaas, S. & Futter, M. Pollution: do microplastics spill on to farm soils? Nature 537, 488 (2016).
Duis, K. & Coors, A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 28, 2 (2016).
Besseling, E., Wang, B., Lurling, M. & Koelmans, A. A. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 48, 12336–12343 (2014).
della Torre, C. et al. Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environ. Sci. Technol. 48, 12302–12311 (2014).
Rillig, M. C., Lehmann, A., de Souza Machado, A. A. & Yang, G. Microplastic effects on plants. New Phytol. 223, 1066–1070 (2019).
Rochman, C. M. Microplastics research—from sink to source. Science 360, 28–29 (2018).
Scheurer, M. & Bigalke, M. Microplastics in Swiss floodplain soils. Environ. Sci. Technol. 52, 3591–3598 (2018).
Zubris, K. A. & Richards, B. K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 138, 201–211 (2005).
Fuller, S. & Gautam, A. A procedure for measuring microplastics using pressurized fluid extraction. Environ. Sci. Technol. 50, 5774–5780 (2016).
Etxeberria, E., Gonzalez, P., Baroja-Fernandez, E. & Romero, J. P. Fluid phase endocytic uptake of artificial nano-spheres and fluorescent quantum dots by sycamore cultured cells: evidence for the distribution of solutes to different intracellular compartments. Plant Signal. Behav. 1, 196–200 (2006).
Huang, C. et al. Transformation of 14C-labeled graphene to 14CO2 in the shoots of a rice plant. Angew. Chem. Int. Ed. 130, 9907–9911 (2018).
Slomberg, D. L. & Schoenfisch, M. H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 46, 10247–10254 (2012).
Wang, Z. et al. CuO nanoparticle interaction with Arabidopsis thaliana: toxicity, parent-progeny transfer, and gene expression. Environ. Sci. Technol. 50, 6008–6016 (2016).
Zhang, M., Ellis, E. A., Cisneros-Zevallos, L. & Akbulut, M. Uptake and translocation of polymeric nanoparticulate drug delivery systems into ryegrass. RSC Adv. 2, 9679–9686 (2012).
Rice-Evans, C., Miller, N. & Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 2, 152–159 (1997).
Paré, P. W. & Tumlinson, J. H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 121, 325–332 (1999).
Dodd, A. N. et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633 (2005).
Tsukagoshi, H., Busch, W. & Benfey, P. N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143, 606–616 (2010).
García-Sánchez, S., Bernales, I. & Cristobal, S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genom. 16, 341 (2015).
Pan, J. W. et al. Root border cell development is a temperature-insensitive and Al-sensitive process in barley. Plant Cell Physiol. 45, 751–760 (2004).
Liu, Q. et al. Study of the inhibitory effect of water-soluble fullerenes on plant growth at the cellular level. ACS Nano 4, 5743–5748 (2010).
Ma, C., White, J. C., Dhankher, O. P. & Xing, B. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ. Sci. Technol. 49, 7109–7122 (2015).
Wang, Z. et al. Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 46, 4434–4441 (2012).
Mitrano, D. M. et al. Synthesis of metal-doped nanoplastics and their utility to investigate fate and behaviour in complex environmental systems. Nat. Nanotechnol. 14, 362–368 (2019).
Avellan, A. et al. Nanoparticle uptake in plants: gold nanomaterial localized in roots of Arabidopsis thaliana by X-ray computed nanotomography and hyperspectral imaging. Environ. Sci. Technol. 51, 8682–8691 (2017).
Klug, B. & Horst, W. J. Oxalate exudation into the root-tip water free space confers protection from aluminum toxicity and allows aluminum accumulation in the symplast in buckwheat (Fagopyrum esculentum). New Phytol. 187, 380–391 (2010).
Feng, L. J. et al. Role of extracellular polymeric substances in the acute inhibition of activated sludge by polystyrene nanoparticles. Environ. Pollut. 238, 859–865 (2018).
Holzapfel, V., Musyanovych, A., Landfester, K., Lorenz, M. R. & Mailänder, V. Preparation of fluorescent carboxyl and amino functionalized polystyrene particles by miniemulsion polymerization as markers for cells. Macromol. Chem. Phys. 206, 2440–2449 (2005).
Feng, L. J. et al. Short-term exposure to positively charged polystyrene nanoparticles causes oxidative stress and membrane destruction in cyanobacteria. Environ. Sci. Nano 6, 3072–3079 (2019).
Wintermans, J. F. G. M. & de Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. Biochim. Biophys. Acta Biophys. Incl. Photsynth. 109, 448–453 (1965).
Napsucialy-Mendivil, S., Alvarez-Venegas, R., Shishkova, S. & Dubrovsky, J. G. Arabidopsis homolog of trithorax1 (ATX1) is required for cell production, patterning, and morphogenesis in root development. J. Exp. Bot. 65, 6373–6384 (2014).
Liu, X. et al. Arsenic induced phytate exudation, and promoted FeAsO4 dissolution and plant growth in As-hyperaccumulator Pteris vittata. Environ. Sci. Technol. 50, 9070–9077 (2016).
Kong, X. et al. PHB3 maintains root stem cell niche identity through ROS-responsive AP2/ERF transcription factors in Arabidopsis. Cell Rep. 22, 1350–1363 (2018).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21776163, 21676161 and U196224), Shandong Provincial Natural Science Foundation (ZR2019JQ18), Youth Interdisciplinary Science and Innovative Research Groups of Shandong University (2020QNQT014), the Fundamental Research Funds of Shandong University (2017JC021), the Qilu Youth Talent Program of Shandong University, the USDA-NIFA Hatch program (MAS 00549) and the UMass Amherst Conti Fellowship.
Author information
Authors and Affiliations
Contributions
X.-Z.Y., S.-G.W. and B.X. designed the study. X.-Z.Y. and X.-D.S. wrote the manuscript. X.-Z.Y. and X.-D.S. analysed the results. Y.-B.J., H.T., X.K. and L.-J.F. performed the transcriptomics experiments. Y.-B.J. and J.-J.L. contributed to histological stains. F.-P.Z. contributed to high-performance liquid chromatography analyses. J.-L.D. and S.-S.D. synthesized nanoplastics. X.-Z.Y., X.-D.S., S.-G.W. and B.X. evaluated and revised the manuscript. Z.D. proofread the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Catherine Santaella, Geraldine Sarret and Fabienne Schwab for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–8, Figs. 1–15 and refs. 1–22.
Rights and permissions
About this article
Cite this article
Sun, XD., Yuan, XZ., Jia, Y. et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 15, 755–760 (2020). https://doi.org/10.1038/s41565-020-0707-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0707-4
This article is cited by
-
Nanoplastics as Trojan Horses: Deciphering Complex Connections and Environmental Ramifications: A Review
Chemistry Africa (2024)
-
Toxicity mechanisms of photodegraded polyvinyl chloride nanoplastics on pea seedlings
Frontiers of Environmental Science & Engineering (2024)
-
Microplastic contamination in the agricultural soil—mitigation strategies, heavy metals contamination, and impact on human health: a review
Plant Cell Reports (2024)
-
Food chain microplastics contamination and impact on human health: a review
Environmental Chemistry Letters (2024)
-
Advances in Physiological and Ecological Effects of Microplastic on Crop
Journal of Soil Science and Plant Nutrition (2024)