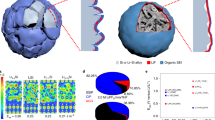
Abstract
High-capacity alloy anode materials for Li-ion batteries have long been held back by limited cyclability caused by the large volume changes during lithium insertion and removal. Hollow and yolk-shell nanostructures have been used to increase the cycling stability by providing an inner void space to accommodate volume changes and a mechanically and dimensionally stable outer surface. These materials, however, require complex synthesis procedures. Here, using in situ transmission electron microscopy, we show that sufficiently small antimony nanocrystals spontaneously form uniform voids on the removal of lithium, which are then reversibly filled and vacated during cycling. This behaviour is found to arise from a resilient native oxide layer that allows for an initial expansion during lithiation but mechanically prevents shrinkage as antimony forms voids during delithiation. We developed a chemomechanical model that explains these observations, and we demonstrate that this behaviour is size dependent. Thus, antimony naturally evolves to form optimal nanostructures for alloy anodes, as we show through electrochemical experiments in a half-cell configuration in which 15-nm antimony nanocrystals have a consistently higher Coulombic efficiency than larger nanoparticles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this article and other findings of this study are available from the corresponding author upon reasonable request.
References
Nitta, N., Wu, F., Lee, J. T. & Yushin, G. Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015).
Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 196, 13–24 (2011).
Schmuch, R., Wagner, R., Hörpel, G., Placke, T. & Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 3, 267–278 (2018).
Obrovac, M. N. & Chevrier, V. L. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 114, 11444–11502 (2014).
Chan, C. K. et al. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 3, 31–35 (2008).
McDowell, M. T., Lee, S. W., Nix, W. D. & Cui, Y. 25th anniversary article: understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 25, 4966–4985 (2013).
McDowell, M. T., Xia, S. & Zhu, T. The mechanics of large-volume-change transformations in high-capacity battery materials. Extreme Mech. Lett. 9, 480–494 (2016).
Liu, N. et al. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 9, 187–192 (2014).
Lee, S. W., McDowell, M. T., Berla, L. A., Nix, W. D. & Cui, Y. Fracture of crystalline silicon nanopillars during electrochemical lithium insertion. Proc. Natl Acad. Sci. USA 109, 4080–4085 (2012).
Chen, Q. & Sieradzki, K. Spontaneous evolution of bicontinuous nanostructures in dealloyed Li-based systems. Nat. Mater. 12, 1102–1106 (2013).
Boebinger, M. G. et al. Avoiding fracture in a conversion battery material through reaction with larger ions. Joule 2, 1783–1799 (2018).
Wang, J. et al. Structural evolution and pulverization of tin nanoparticles during lithiation–delithiation cycling. J. Electrochem. Soc. 161, F3019–F3024 (2014).
Ebner, M., Marone, F., Stampanoni, M. & Wood, V. Visualization and quantification of electrochemical and mechanical degradation in Li ion batteries. Science 342, 716–720 (2013).
Qi, W. et al. Nanostructured anode materials for lithium-ion batteries: principle, recent progress and future perspectives. J. Mater. Chem. A 5, 19521–19540 (2017).
Zhang, S., Zhao, K., Zhu, T. & Li, J. Electrochemomechanical degradation of high-capacity battery electrode materials. Prog. Mater. Science 89, 479–521 (2017).
Wu, H. et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 7, 310–315 (2012).
Liu, N. et al. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 12, 3315–3321 (2012).
Liu, J. et al. New nanoconfined galvanic replacement synthesis of hollow Sb@C yolk-shell spheres constituting a stable anode for high-rate Li/Na-ion batteries. Nano Lett. 17, 2034–2042 (2017).
Liu, S., Feng, J., Bian, X., Liu, J. & Xu, H. The morphology-controlled synthesis of a nanoporous-antimony anode for high-performance sodium-ion batteries. Energy Environ. Sci. 9, 1229–1236 (2016).
Liang, W. et al. Nanovoid formation and annihilation in gallium nanodroplets under lithiation–delithiation cycling. Nano Lett. 13, 5212–5217 (2013).
Zhou, X. et al. In situ focused ion beam scanning electron microscope study of microstructural evolution of single tin particle anode for Li-ion batteries. ACS Appl. Mater. Interfaces 11, 1733–1738 (2019).
Adkins, E. R., Jiang, T., Luo, L., Wang, C.-M. & Korgel, B. A. In situ transmission electron microscopy of oxide shell-induced pore formation in (de)lithiated silicon nanowires. ACS Energy Lett. 3, 2829–2834 (2018).
He, Y. et al. In situ transmission electron microscopy probing of native oxide and artificial layers on silicon nanoparticles for lithium ion batteries. ACS Nano 8, 11816–11823 (2014).
Liu, X. H. et al. Reversible nanopore formation in Ge nanowires during lithiation–delithiation cycling: an in situ transmission electron microscopy study. Nano Lett. 11, 3991–3997 (2011).
He, M., Kravchyk, K., Walter, M. & Kovalenko, M. V. Monodisperse antimony nanocrystals for high-rate Li-ion and Na-ion battery anodes: nano versus bulk. Nano Lett. 14, 1255–1262 (2014).
Darwiche, A. et al. Better cycling performances of bulk Sb in Na-ion batteries compared to Li-ion systems: an unexpected electrochemical mechanism. J. Am. Chem. Soc. 134, 20805–20811 (2012).
Baggetto, L. et al. Intrinsic thermodynamic and kinetic properties of Sb electrodes for Li-ion and Na-ion batteries: experiment and theory. J. Mater. Chem. A 1, 7985–7994 (2013).
Li, Z. et al. Coupling in situ TEM and ex situ analysis to understand heterogeneous sodiation of antimony. Nano Lett. 15, 6339–6348 (2015).
Aricò, A. S., Bruce, P., Scrosati, B., Tarascon, J.-M. & van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 4, 366–377 (2005).
Huang, J. Y. et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science 330, 1515–1520 (2010).
Gutiérrez-Kolar, J. S. et al. Interpreting electrochemical and chemical sodiation mechanisms and kinetics in tin antimony battery anodes using in situ transmission electron microscopy and computational methods. ACS Appl. Energy Mater. 2, 3578–3586 (2019).
Ruzmetov, D. et al. Electrolyte stability determines scaling limits for solid-state 3D Li ion batteries. Nano Lett. 12, 505–511 (2012).
Xie, H. et al. β-SnSb for sodium ion battery anodes: phase transformations responsible for enhanced cycling stability revealed by in situ TEM. ACS Energy Lett. 3, 1670–1676 (2018).
He, K. et al. Visualizing non-equilibrium lithiation of spinel oxide via in situ transmission electron microscopy. Nat. Commun. 7, 11441 (2016).
Boebinger, M. G. et al. Distinct nanoscale reaction pathways in a sulfide material for sodium and lithium batteries. J. Mater. Chem. A 5, 11701–11709 (2017).
McDowell, M. T. et al. In situ TEM of two-phase lithiation of amorphous silicon nanospheres. Nano Lett. 13, 758–764 (2013).
Goodno, B. J. & Gere, J. M. Statics and Mechanics of Materials (Cengage, 2019).
Hutchinson, J. W. Buckling of spherical shells revisited. Proc. R. Soc. A 472, 20160577 (2016).
Niu, K.-Y., Park, J., Zheng, H. & Alivisatos, A. P. Revealing bismuth oxide hollow nanoparticle formation by the Kirkendall effect. Nano Lett. 13, 5715–5719 (2013).
Wang, W., Dahl, M. & Yin, Y. Hollow nanocrystals through the nanoscale Kirkendall effect. Chem. Mater. 25, 1179–1189 (2013).
Arganda-Carreras, I. et al. Trainable Weka Segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics 33, 2424–2426 (2017).
Tippens, J. et al. Visualizing chemomechanical degradation of a solid-state battery electrolyte. ACS Energy Lett. 4, 1475–1483 (2019).
Bowen, J. R. DiffractIndex (Matlab Central File Exchange, accessed 29 October 2018); https://www.mathworks.com/matlabcentral/fileexchange/54789-diffractindex
Kravchyk, K. et al. Monodisperse and inorganically capped Sn and Sn/SnO2 nanocrystals for high-performance Li-ion battery anodes. J. Am. Chem. Soc. 135, 4199–4202 (2013).
Acknowledgements
This work was performed at the Georgia Tech Materials Characterization Facility and the Institute for Electronics and Nanotechnology, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (Grant ECCS-1542174). M.G.B. acknowledges support from the DOE Office of Science Graduate Student Research Program for research performed at Oak Ridge National Laboratory. A portion of this research was conducted at the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility (K.A.U. and R.R.U.). M.T.M. acknowledges support from a Sloan Research Fellowship in Chemistry from the Alfred P. Sloan Foundation. M.Y. acknowledges financial support from the Swiss National Science foundation via an Ambizione Fellowship (no. 161249). M.G.B. acknowledges F. J. Q. Cortes for assistance with the Cr deposition and N. Kondekar for assistance with the diffraction analysis.
Author information
Authors and Affiliations
Contributions
M.T.M., V.W. and M.G.B. conceived the study. M.G.B. conducted the TEM experiments, electrochemical experiments and data analysis. O.Y., M.Y. and V.W. synthesized all the materials used for this study and contributed to the data analysis. K.A.U. conducted the STEM–EDS experiments. R.R.U. assisted with the TEM and STEM measurements. M.T.M. guided the experiments and data analysis and performed modelling. M.G.B. and M.T.M. wrote the manuscript with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Morphology evolution of a group of Sb nanocrystals during repeated cycling.
(a) Group of pristine Sb crystals before cycling. (b) The same cluster after the first lithiation (i) and delithiation (ii). (c) After the second lithiation/delithiation. (d) After the third lithiation/delithiation. (e) After the fourth lithiation/delithiation. (f) After the fifth lithiation/delithiation. (g) After the sixth lithiation/delithiation. Since this is a larger group of particles compared to Supplementary Fig. 3, some particles are not fully lithiated or delithiated in each cycle due to transport limitations in the in situ experiment, resulting in a lack of volume change or hollowing behavior during that particular cycle.
Extended Data Fig. 2 Galvanostatic curves from Sb-based electrodes with different particle sizes at two different rates: 1 C (660 mA g−1) and C/10 (66 mA g−1).
(a, b) Data from 15-nm monodisperse nanocrystals at C/10 (a) and 1 C (b). (c, d) Data from 40-140 nm polydisperse nanoparticles at C/10 (c) and 1 C (d). (e, f) Data from bulk particles at C/10 (e) and 1 C (f). The curves at a rate of 1 C correspond to the specific capacity plots in Fig. 4b in the main text. All three samples at both rates show a discharge plateau at ~0.8 V vs. Li/Li+ and a charge plateau at ~1.0 V vs. Li/Li+, which are ascribed to the two-phase lithiation/delithiation of Sb. The small nanocrystals and larger nanoparticles show a higher plateau (~0.8–1.4 V vs. Li/Li+) during the initial discharge that corresponds to the conversion of the surface oxides to a lithiated phase (a, c). The nanocrystals exhibit a greater specific capacity associated with this oxide layer reaction (a) than the larger nanoparticles (c) due to the greater surface area of the nanocrystals.
Extended Data Fig. 3 Comparing in situ TEM sodiation/desodiation of Sb nanocrystals to lithiation/delithiation.
(a) TEM image of a group of Sb nanocrystals that have undergone sodiation. (b) Image of a different group of Sb nanocrystals that have been desodiated. (c) TEM image of a group of Sb nanocrystals that have undergone lithiation, and (d) the same group after delithiation. The morphology of the delithiated and desodiated particles is similar. In these images, it is clear that there is some merging of the oxide shells between particles after the reaction process, which is especially clear for the desodiation case (b). The metal regions within the oxide shells remain distinct, however.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15, Videos 1 and 2, Methods and refs. 1–3.
Supplementary Video 1
Supplementary Video 1 shows the volume expansion during the initial lithiation process of a group of Sb nanocrystals. This video is shown at 4 times the actual speed.
Supplementary Video 2
Supplementary Video 2 shows several lithiation and delithiation steps that the Sb nanocrystals in Fig. 2 underwent, starting with the second lithiation of the particles (the first cycle was not captured on video). This is followed by the delithiation process that is discussed and shown in detail in Fig. 2. Finally, the third lithiation step is shown as the voids are filled once more. This video is shown at 6 times the actual speed.
Source data
Source Data Fig. 2
Data used for plotting Fig. 2e.
Source Data Fig. 3
Data used for plotting Fig. 3a.
Source Data Fig. 4
Data used for plotting Fig. 4.
Source Data Extended Data Fig. 2
Data use for plotting Extended Data Fig. 2.
Rights and permissions
About this article
Cite this article
Boebinger, M.G., Yarema, O., Yarema, M. et al. Spontaneous and reversible hollowing of alloy anode nanocrystals for stable battery cycling. Nat. Nanotechnol. 15, 475–481 (2020). https://doi.org/10.1038/s41565-020-0690-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0690-9
This article is cited by
-
Interfacial carbon tailoring of porous SiOx/carbon nanotube hybrids towards high-rate lithium storage
Discover Applied Sciences (2024)
-
Template-free synthesis of hollow carbon-based nanostructures from MOFs for rechargeable battery applications
Science China Chemistry (2023)
-
Effect of crystallite geometries on electrochemical performance of porous intercalation electrodes by multiscale operando investigation
Nature Materials (2022)
-
Nano-vault architecture mitigates stress in silicon-based anodes for lithium-ion batteries
Communications Materials (2021)
-
Spontaneous void formation in antimony alloy anodes
Communications Chemistry (2020)