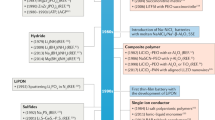
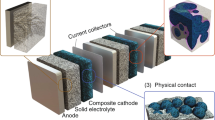
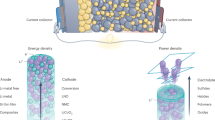
Abstract
The recent discovery of highly conductive solid-state electrolytes (SSEs) has led to tremendous progress in the development of all-solid-state batteries (ASSBs). Though promising, they still face barriers that limit their practical application, such as poor interfacial stability, scalability challenges and production safety. Additionally, efforts to develop sustainable manufacturing of lithium ion batteries are still lacking, with no prevailing strategy developed yet to handle recyclability of ASSBs. To date, most SSE research has been largely focused on the discovery of novel electrolytes. Recent review articles have extensively examined a broad spectrum of these SSEs using evaluation factors such as conductivity and chemical stability. Recognizing this, in this Review we seek to evaluate SSEs beyond conventional factors and offer a perspective on various bulk, interface and nanoscale phenomena that require urgent attention within the scientific community. We provide a realistic assessment of the current state-of-the-art characterization techniques and evaluate future full cell ASSB prototyping strategies. We hope to offer rational solutions to overcome some major fundamental obstacles faced by the ASSB community, as well as potential strategies toward a sustainable ASSB recycling model.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
05 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41565-021-00877-5
References
Yu, C. et al. Facile synthesis toward the optimal structure-conductivity characteristics of the argyrodite Li6PS5Cl solid-state electrolyte. ACS Appl. Mater. Interfaces 10, 33296–33306 (2018).
Nguyen, H. et al. Single-step synthesis of highly conductive Na3PS4 solid electrolyte for sodium all solid-state batteries. J. Power Sources 435, 126623 (2019).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Zhu, Y., He, X. & Mo, Y. Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 7, 23685–23693 (2015).
Zhang, H. et al. Single lithium-ion conducting solid polymer electrolytes: advances and perspectives. Chem Soc Rev 46, 797–815 (2017).
Mindemark, J., Lacey, M. J., Bowden, T. & Brandell, D. Beyond PEO—alternative host materials for Li+ conducting solid polymer electrolytes. Prog. Polym. Sci. 81, 114–143 (2018).
Yao, P. et al. Review on polymer-based composite electrolytes for lithium batteries. Front. Chem. 7, 522 (2019).
Auvergniot, J. et al. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem. Mater. 29, 3883–3890 (2017).
Auvergniot, J. et al. Redox activity of argyrodite Li6PS5Cl electrolyte in all-solid-state Li-ion battery: an XPS study. Solid State Ionics 300, 78–85 (2017).
Haruyama, J., Sodeyama, K., Han, L., Takada, K. & Tateyama, Y. Space–charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery. Chem. Mater. 26, 4248–4255 (2014).
Ohta, N. et al. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification. Adv. Mater. 18, 2226–2229 (2006).
Li, X. et al. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery. J. Energy Chem. 40, 39–45 (2019).
Haruyama, J., Sodeyama, K. & Tateyama, Y. Cation mixing properties toward Co diffusion at the LiCoO2 cathode/sulfide electrolyte interface in a solid-state battery. ACS Appl. Mater. Interfaces 9, 286–292 (2017).
Sakuda, A., Hayashi, A. & Tatsumisago, M. Interfacial observation between LiCoO2 electrode and li2s−p2s5 solid electrolytes of all-solid-state lithium secondary batteries using transmission electron microscopy. Chem. Mater. 22, 949–956 (2010).
Woo, J. H. et al. Nanoscale interface modification of LiCoO2 by Al2O3 atomic layer deposition for solid-state li batteries. J. Electrochem. Soc. 159, A1120–A1124 (2012).
Okada, K. et al. Preparation and electrochemical properties of LiAlO2 coated Li(Ni1/3Mn1/3Co1/3)O2 for all-solid-state batteries. Solid State Ionics 255, 120–127 (2014).
Xiao, Y., Miara, L. J., Wang, Y. & Ceder, G. Computational Screening of Cathode Coatings for Solid-State. Batteries. Joule 3, 1252–1275 (2019).
Koerver, R. et al. Capacity fade in solid-state batteries: interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes. Chem. Mater. 29, 5574–5582 (2017).
Wenzel, S. et al. Direct observation of the interfacial instability of the fast ionic conductor Li10GeP2S12 at the lithium metal anode. Chem. Mater. 28, 2400–2407 (2016).
Han, X. et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017).
Yersak, T., Salvador, J. R., Schmidt, R. D. & Cai, M. Hot pressed, fiber-reinforced (Li2S)70(P2S5)30 solid-state electrolyte separators for Li metal batteries. ACS Appl. Energy Mater. 2, 3523–3531 (2019).
Kim, S. H. et al. In situ observation of lithium metal plating in a sulfur-based solid electrolyte for all-solid-state batteries. J. Mater. Chem. A 7, 13650–13657 (2019).
Tao, X. et al. Solid-state lithium-sulfur batteries operated at 37 °C with composites of nanostructured Li7La3Zr2O12/carbon foam and polymer. Nano Lett. 17, 2967–2972 (2017).
Ates, T., Keller, M., Kulisch, J., Adermann, T. & Passerini, S. Development of an all-solid-state lithium battery by slurry-coating procedures using a sulfidic electrolyte. Energy Storage Mater. 17, 204–210 (2019).
Han, F., Yue, J., Zhu, X. & Wang, C. Suppressing Li dendrite formation in Li2S-P2S5 solid electrolyte by LiI incorporation. Adv. Energy Mater. 8, 1703644 (2018).
Li, Y. et al. Mastering the interface for advanced all-solid-state lithium rechargeable batteries. Proc. Natl Acad. Sci. USA 113, 13313–13317 (2016).
Kerman, K., Luntz, A., Viswanathan, V., Chiang, Y.-M. & Chen, Z. Review—practical challenges hindering the development of solid state Li ion batteries. J. Electrochem. Soc. 164, A1731–A1744 (2017).
Aguesse, F. et al. Investigating the dendritic growth during full cell cycling of garnet electrolyte in direct contact with Li metal. ACS Appl. Mater. Interfaces 9, 3808–3816 (2017).
Swamy, T. et al. Lithium metal penetration induced by electrodeposition through solid electrolytes: example in single-crystal Li6La3ZrTaO12 garnet. J. Electrochem. Soc. 165, A3648–A3655 (2018).
Neudecker, B. J., Dudney, N. J. & Bates, J. B. “Lithium-free” thin-film battery with in situ plated Li snode. J. Electrochem. Soc. 147, 517–523 (2000).
Li, J., Ma, C., Chi, M., Liang, C. & Dudney, N. J. Solid electrolyte: the key for high-voltage lithium batteries. Adv. Energy Mater. 5, 1401408 (2015).
Fang, C. et al. Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019).
Sharafi, A. et al. Surface chemistry mechanism of ultra-low interfacial resistance in the solid-state electrolyte Li7La3Zr2O12. Chem. Mater. 29, 7961–7968 (2017).
Tao, Y. et al. Lithium superionic conducting oxysulfide solid electrolyte with excellent stability against lithium metal for all-solid-state cells. J. Electrochem. Soc. 163, A96–A101 (2016).
Sun, Y. et al. Oxygen substitution effects in Li10GeP2S12 solid electrolyte. J. Power Sources 324, 798–803 (2016).
Asano, T. et al. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries. Adv. Mater. 30, e1803075 (2018).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Han, F., Zhu, Y., He, X., Mo, Y. & Wang, C. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv. Energy Mater. 6, 1501590 (2016).
Han, F., Gao, T., Zhu, Y., Gaskell, K. J. & Wang, C. A battery made from a single material. Adv. Mater. 27, 3473–3483 (2015).
Yan, X., Li, Z., Wen, Z. & Han, W. Li/Li7La3Zr2O12/LiFePO4 All-solid-state battery with ultrathin nanoscale solid electrolyte. J. Phys. Chem. C 121, 1431–1435 (2017).
Shi, X. et al. Fabrication and electrochemical properties of LATP/PVDF composite electrolytes for rechargeable lithium-ion battery. Solid State Ion. 325, 112–119 (2018).
Zhang, W. et al. The detrimental effects of carbon additives in Li10GeP2S12 based solid-state batteries. ACS Appl. Mater. Interfaces 9, 35888–35896 (2017).
Xu, L. et al. Interfaces in solid-state lithium batteries. Joule 2, 1991–2015 (2018).
Lewis, J. A., Tippens, J., Cortes, F. J. Q. & McDowell, M. T. Chemo-mechanical challenges in solid-state batteries. Trends Chem. 1, 845–857 (2019).
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Ren, Y., Shen, Y., Lin, Y. & Nan, C.-W. Direct observation of lithium dendrites inside garnet-type lithium-ion solid electrolyte. Electrochem. Commun. 57, 27–30 (2015).
Seitzman, N. et al. Toward all-solid-state lithium batteries: three-dimensional visualization of lithium migration in β-Li3PS4 ceramic electrolyte. J. Electrochem. Soc. 165, A3732–A3737 (2018).
Li, T. et al. Three-dimensional reconstruction and analysis of all-solid Li-ion battery electrode using synchrotron transmission X-ray microscopy tomography. ACS Appl. Mater. Interfaces 10, 16927–16931 (2018).
Chen-Wiegart, Y.-cK., Liu, Z., Faber, K. T., Barnett, S. A. & Wang, J. 3D analysis of a LiCoO2–Li(Ni1/3Mn1/3Co1/3)O2 Li-ion battery positive electrode using x-ray nano-tomography. Electrochem. Commun. 28, 127–130 (2013).
Wang, C. et al. In situ neutron depth profiling of lithium metal-garnet interfaces for solid state batteries. J. Am. Chem. Soc. 139, 14257–14264 (2017).
Marbella, L. E. et al. 7Li NMR chemical shift imaging to detect microstructural growth of lithium in all-solid-state batteries. Chem. Mater. 31, 2762–2769 (2019).
Lee, J. Z. et al. Cryogenic focused ion beam characterization of lithium metal anodes. ACS Energy Lett. 4, 489–493 (2019).
Choi, S. et al. Quantitative analysis of microstructures and reaction interfaces on composite cathodes in all-solid-state batteries using a three-dimensional reconstruction technique. ACS Appl. Mater. Interfaces 10, 23740–23747 (2018).
Santhanagopalan, D. et al. Interface limited lithium transport in solid-state batteries. J. Phys. Chem. Lett. 5, 298–303 (2014).
Hakari, T. et al. Structural and electronic-state changes of a sulfide solid electrolyte during the Li deinsertion–insertion processes. Chem. Mater. 29, 4768–4774 (2017).
Schnell, J. et al. All-solid-state lithium-ion and lithium metal batteries – paving the way to large-scale production. J. Power Sources 382, 160–175 (2018).
Zhang, X. et al. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J. Am. Chem. Soc. 139, 13779–13785 (2017).
Jung, Y.-C., Lee, S.-M., Choi, J.-H., Jang, S. S. & Kim, D.-W. All Solid-State Lithium Batteries Assembled with Hybrid Solid Electrolytes. J. Electrochem. Soc. 162, A704–A710 (2015).
Nam, Y. J., Oh, D. Y., Jung, S. H. & Jung, Y. S. Toward practical all-solid-state lithium-ion batteries with high energy density and safety: comparative study for electrodes fabricated by dry- and slurry-mixing processes. J. Power Sources 375, 93–101 (2018).
Tan, D. H. S. et al. Enabling thin and flexible solid-state composite electrolytes by the scalable solution process. ACS Appl. Energy Mater. 2, 6542–6550 (2019).
Sakuda, A. et al. All-solid-state battery electrode sheets prepared by a slurry coating process. J. Electrochem. Soc. 164, A2474–A2478 (2017).
Hippauf, F. et al. Overcoming binder limitations of sheet-type solid-state cathodes using a solvent-free dry-film approach. Energy Storage Mater. 21, 390–398 (2019).
Lee, K. et al. Selection of binder and solvent for solution-processed all-solid-state battery. J. Electrochem. Soc. 164, A2075–A2081 (2017).
Lee, K., Lee, J., Choi, S., Char, K. & Choi, J. W. Thiol–ene click reaction for fine polarity tuning of polymeric binders in solution-processed all-solid-state batteries. ACS Energy Lett. 4, 94–101 (2018).
Hayashi, A., Muramatsu, H., Ohtomo, T., Hama, S. & Tatsumisago, M. Improvement of chemical stability of Li3PS4 glass electrolytes by adding MxOy (M = Fe, Zn, and Bi) nanoparticles. J. Mater. Chem. A 1, 6320 (2013).
Liang, X., Han, D., Wang, Y., Lan, L. & Mao, J. Preparation and performance study of a PVDF–LATP ceramic composite polymer electrolyte membrane for solid-state batteries. RSC Adv. 8, 40498–40504 (2018).
Oh, D. Y. et al. Excellent compatibility of solvate ionic liquids with sulfide solid electrolytes: toward favorable ionic contacts in bulk-type all-solid-state lithium-ion batteries. Adv. Energy Mater. 5, 1500865 (2015).
Zeng, X., Li, J. & Ren, Y. Prediction of various discarded lithium batteries in china. In Proc. IEEE International Symposium on Sustainable Systems and Technology (ISSST) 1–4 (IEEE, 2012).
Oh, D. Y. et al. Slurry‐fabricable Li+ conductive polymeric binders for practical all‐solid‐state lithium‐ion batteries enabled by solvate ionic liquids. Adv. Energy Mater. 9, 1802927 (2019).
Yamamoto, M., Terauchi, Y., Sakuda, A. & Takahashi, M. Binder-free sheet-type all-solid-state batteries with enhanced rate capabilities and high energy densities. Sci. Rep. 8, 1212 (2018).
Xu, J. et al. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 177, 512–527 (2008).
May, G. J., Davidson, A. & Monahov, B. Lead batteries for utility energy storage: a review. J. Energy Storage 15, 145–157 (2018).
Zhang, X. et al. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 47, 7239–7302 (2018).
Li, L. et al. The recycling of spent lithium-ion batteries: a review of current processes and technologies. Electrochem. Energy Rev. 1, 461–482 (2018).
Liu, T. et al. Sustainability-inspired cell design for a fully recyclable sodium ion battery. Nat. Commun. 10, 1965 (2019).
Zheng, X. et al. A mini-review on metal recycling from spent lithium ion batteries. Engineering 4, 361–370 (2018).
Nowak, S. & Winter, M. The role of sub- and supercritical CO2 as “Processing Solvent” for the recycling and sample preparation of lithium ion battery electrolytes. Molecules 22, 403 (2017).
Miura, A. et al. Liquid-phase syntheses of sulfide electrolytes for all-solid-state lithium battery. Nat. Rev. Chem. 3, 189–198 (2019).
Wang, Y. et al. Mechanism of formation of Li7P3S11 solid electrolytes through liquid phase synthesis. Chem. Mater. 30, 990–997 (2018).
Kim, D. H. et al. Infiltration of solution-processable solid electrolytes into conventional Li-Ion-battery electrodes for all-solid-state Li-Ion batteries. Nano Lett 17, 3013–3020 (2017).
Calpa, M., Rosero-Navarro, N. C., Miura, A. & Tadanaga, K. Instantaneous preparation of high lithium-ion conducting sulfide solid electrolyte Li7P3S11 by a liquid phase process. RSC Adv. 7, 46499–46504 (2017).
Jung, S.-K. et al. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 4, 1300787 (2014).
Zhang, X. et al. sustainable recycling and regeneration of cathode scraps from industrial production of lithium-ion batteries. ACS Sustainable Chem. Eng. 4, 7041–7049 (2016).
Shi, Y., Zhang, M., Meng, Y. S. & Chen, Z. Ambient‐pressure relithiation of degraded LixNi0.5Co0.2Mn0.3O2 (0 < x < 1) via eutectic solutions for direct regeneration of lithium‐ion battery cathodes. Adv. Energy Mater. 9, 1900454 (2019).
Wang, S. et al. Lithium chlorides and bromides as promising solid-state chemistries for fast ion conductors with good electrochemical stability. Angew. Chem. Int. Ed. 58, 8039–8043 (2019).
Wang, Z. et al. In situ STEM-EELS observation of nanoscale interfacial phenomena in all-solid-state batteries. Nano Lett. 16, 3760–3767 (2016).
Sang, L., Haasch, R. T., Gewirth, A. A. & Nuzzo, R. G. Evolution at the solid electrolyte/gold electrode interface during lithium deposition and stripping. Chem. Mater. 29, 3029–3037 (2017).
Acknowledgements
This work was financially supported by the LG Chem through the Battery Innovation Contest (BIC) program as well as the Energy & Biosciences Institute through the EBI-Shell program. Z.C. acknowledges funding from the US Department of Energy via ReCell Center and the start-up fund support from the Jacob School of Engineering at University of California San Diego. Y.S.M. acknowledges the funding support from Zable Endowed Chair Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, D.H.S., Banerjee, A., Chen, Z. et al. From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries. Nat. Nanotechnol. 15, 170–180 (2020). https://doi.org/10.1038/s41565-020-0657-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0657-x
This article is cited by
-
Recycling of solid-state batteries
Nature Energy (2024)
-
Li–Solid Electrolyte Interfaces/Interphases in All-Solid-State Li Batteries
Electrochemical Energy Reviews (2024)
-
Mn-based cathode materials for rechargeable batteries
Science China Chemistry (2024)
-
Collaborative and privacy-preserving retired battery sorting for profitable direct recycling via federated machine learning
Nature Communications (2023)
-
A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries
Nature Communications (2023)