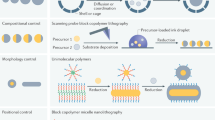
Abstract
Confining molecules can fundamentally change their chemical and physical properties. Confinement effects are considered instrumental at various stages of the origins of life, and life continues to rely on layers of compartmentalization to maintain an out-of-equilibrium state and efficiently synthesize complex biomolecules under mild conditions. As interest in synthetic confined systems grows, we are realizing that the principles governing reactivity under confinement are the same in abiological systems as they are in nature. In this Review, we categorize the ways in which nanoconfinement effects impact chemical reactivity in synthetic systems. Under nanoconfinement, chemical properties can be modulated to increase reaction rates, enhance selectivity and stabilize reactive species. Confinement effects also lead to changes in physical properties. The fluorescence of light emitters, the colours of dyes and electronic communication between electroactive species can all be tuned under confinement. Within each of these categories, we elucidate design principles and strategies that are widely applicable across a range of confined systems, specifically highlighting examples of different nanocompartments that influence reactivity in similar ways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mann, S. The origins of life: Old problems, new chemistries. Angew. Chem. Int. Ed. 52, 155–162 (2013).
Muñoz-Santiburcio, D. & Marx, D. Chemistry in nanoconfined water. Chem. Sci. 8, 3444–3452 (2017).
Lambert, J. B., Gurusamy-Thangavelu, S. A. & Ma, K. The silicate-mediated formose reaction: Bottom-up synthesis of sugar silicates. Science 327, 984–986 (2010).
Ohara, S., Kakegawa, T. & Nakazawa, H. Pressure effects on the abiotic polymerization of glycine. Orig. Life Evol. Biosph. 37, 215–223 (2007).
Hansma, H. G. The power of crowding for the origins of life. Orig. Life Evol. Biosph. 44, 307–311 (2014).
Dzieciol, A. J. & Mann, S. Designs for life: Protocell models in the laboratory. Chem. Soc. Rev. 41, 79–85 (2012).
Koga, S., Williams, D. S., Perriman, A. W. & Mann, S. Peptide–nucleotide microdroplets as a step towards a membrane-free protocell model. Nat. Chem. 3, 720–724 (2011).
Szostak, J. W., Bartel, D. P. & Luisi, P. L. Synthesizing life. Nature 409, 387–390 (2001).
Urban, P. L. Compartmentalised chemistry: From studies on the origin of life to engineered biochemical systems. New J. Chem. 38, 5135–5141 (2014).
Zhou, H.-X. & Dill, K. A. Stabilization of proteins in confined spaces. Biochemistry 40, 11289–11293 (2001).
Panganiban, B. et al. Random heteropolymers preserve protein function in foreign environments. Science 359, 1239–1243 (2018).
Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 14, 630–642 (2013).
Pieters, B. J. G. E., van Eldijk, M. B., Nolte, R. J. M. & Mecinović, J. Natural supramolecular protein assemblies. Chem. Soc. Rev. 45, 24–39 (2016).
Tripp, B. C., Smith, K. & Ferry, J. G. Carbonic anhydrase: New insights for an ancient enzyme. J. Biol. Chem. 276, 48615–48618 (2001).
Shichida, Y. & Matsuyama, T. Evolution of opsins and phototransduction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364, 2881–2895 (2009).
Dutta, S. et al. Structure of a modular polyketide synthase. Nature 510, 512–517 (2014).
Kartal, B. et al. Molecular mechanism of anaerobic ammonium oxidation. Nature 479, 127–130 (2011).
Yan, L. et al. Magnetotactic bacteria, magnetosomes and their application. Microbiol. Res. 167, 507–519 (2012).
Aizenberg, J., Tkachenko, A., Weiner, S., Addadi, L. & Hendler, G. Calcitic microlenses as part of the photoreceptor system in brittlestars. Nature 412, 819–822 (2001).
Grzybowski, B. A. & Huck, W. T. S. The nanotechnology of life-inspired systems. Nat. Nanotechnol. 11, 585–592 (2016).
Yoshizawa, M., Klosterman, J. K. & Fujita, M. Functional molecular flasks: New properties and reactions within discrete, self-assembled hosts. Angew. Chem. Int. Ed. 48, 3418–3438 (2009).
Inokuma, Y., Kawano, M. & Fujita, M. Crystalline molecular flasks. Nat. Chem. 3, 349–358 (2011).
Fujita, M. et al. Self-assembly of ten molecules into nanometre-sized organic host frameworks. Nature 378, 469–471 (1995).
Fujita, D. et al. Self-assembly of tetravalent Goldberg polyhedra from 144 small components. Nature 540, 563–566 (2016).
Cook, T. R. & Stang, P. J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 115, 7001–7045 (2015).
Rebek, J. Molecular behavior in small spaces. Acc. Chem. Res. 42, 1660–1668 (2009).
Liu, L. J. & Rebek, J. in Hydrogen Bonded Supramolecular Structures (eds Li, Z.-T. & Wu, L.-Z.) 227–248 (Springer, 2015).
Aumiller, W. M., Uchida, M. & Douglas, T. Protein cage assembly across multiple length scales. Chem. Soc. Rev. 47, 3433–3469 (2018).
Bode, S. A., Minten, I. J., Nolte, R. J. M. & Cornelissen, J. J. L. M. Reactions inside nanoscale protein cages. Nanoscale 3, 2376–2389 (2011).
Jiang, J., Zhao, Y. & Yaghi, O. M. Covalent chemistry beyond molecules. J. Am. Chem. Soc. 138, 3255–3265 (2016).
Babu, H. V., Bai, M. G. M. & Rajeswara Rao, M. Functional π-conjugated two-dimensional covalent organic frameworks. ACS Appl. Mater. Interfaces 11, 11029–11060 (2019).
Wang, H., Wang, Y., Shen, B., Liu, X. & Lee, M. Substrate-driven transient self-assembly and spontaneous disassembly directed by chemical reaction with product release. J. Am. Chem. Soc. 141, 4182–4185 (2019). Unique example in which substrates drive the self-assembly of a lipid bilayer, which serves as a confined environment for reaction acceleration; the lipid bilayer then disassembles upon product formation.
Zhao, H. et al. Reversible trapping and reaction acceleration within dynamically self-assembling nanoflasks. Nat. Nanotechnol. 11, 82–88 (2015). Empty space between nanoparticles within aggregates is shown to serve as a confined environment for reaction acceleration; guest encapsulation and release can be controlled by the reversible assembly of nanoparticle aggregates.
Fu, Q. & Bao, X. Surface chemistry and catalysis confined under two-dimensional materials. Chem. Soc. Rev. 46, 1842–1874 (2017).
Goronzy, D. P. et al. Supramolecular assemblies on surfaces: Nanopatterning, functionality, and reactivity. ACS Nano 12, 7445–7481 (2018).
Poli, R. (ed) Effects of Nanoconfinement on Catalysis (Springer, 2017).
Brown, C. J., Toste, F. D., Bergman, R. G. & Raymond, K. N. Supramolecular catalysis in metal–ligand cluster hosts. Chem. Rev. 115, 3012–3035 (2015).
Mouarrawis, V., Plessius, R., van der Vlugt, J. I. & Reek, J. N. H. Confinement effects in catalysis using well-defined materials and cages. Front. Chem. 6, 623 (2018).
Leenders, S. H. A. M., Gramage-Doria, R., de Bruin, B. & Reek, J. N. H. Transition metal catalysis in confined spaces. Chem. Soc. Rev. 44, 433–448 (2015).
Liu, X. & Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 1, 16064 (2016).
Pascanu, V., González Miera, G., Inge, A. K. & Martín-Matute, B. Metal–organic frameworks as catalysts for organic synthesis: A critical perspective. J. Am. Chem. Soc. 141, 7223–7234 (2019).
Ennaert, T. et al. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 45, 584–611 (2016).
Turro, N. J., Kraeutler, B. & Anderson, D. R. Magnetic and micellar effects on photoreactions. Micellar cage and magnetic isotope effects on quantum yields. Correlation of 13C enrichment parameters with quantum yield measurements. J. Am. Chem. Soc. 101, 7435–7437 (1979).
Turro, N. J. Micelles, magnets and molecular mechanisms. Application to cage effects and isotope separation. Pure Appl. Chem. 53, 259–286 (1981).
Fendler, J. H. & Fendler, E. J. Catalysis in Micellar and Macromoleular Systems (Academic Press, 1975).
Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 97, 2373–2420 (1997).
Vispute, T. P., Zhang, H., Sanna, A., Xiao, R. & Huber, G. W. Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science 330, 1222–1227 (2010).
Vogt, E. T. C. & Weckhuysen, B. M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 44, 7342–7370 (2015).
Gao, P. et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 9, 1019–1024 (2017).
Snyder, B. E. R. et al. The active site of low-temperature methane hydroxylation in iron-containing zeolites. Nature 536, 317–321 (2016).
Shan, J., Li, M., Allard, L. F., Lee, S. & Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 551, 605–608 (2017).
Morejudo, S. H. et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 353, 563–566 (2016).
Gao, J. et al. Identification of molybdenum oxide nanostructures on zeolites for natural gas conversion. Science 348, 686–690 (2015).
Beale, A. M., Gao, F., Lezcano-Gonzalez, I., Peden, C. H. F. & Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 44, 7371–7405 (2015).
Zhang, R., Liu, N., Lei, Z. & Chen, B. Selective transformation of various nitrogen-containing exhaust gases toward N2 over zeolite catalysts. Chem. Rev. 116, 3658–3721 (2016).
Paolucci, C. et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 357, 898–903 (2017).
Lee, J. K., Samanta, D., Nam, H. G. & Zare, R. N. Micrometer-sized water droplets induce spontaneous reduction. J. Am. Chem. Soc. 141, 10585–10589 (2019). Organic compounds are catalytically reduced under confinement on the inner surface of water microdroplets, demonstrating how similar reactions may have proceeded in the prebiotic era.
Fallah-Araghi, A. et al. Enhanced chemical synthesis at soft interfaces: A universal reaction-adsorption mechanism in microcompartments. Phys. Rev. Lett. 112, 28301 (2014).
Wang, Y. et al. Host–guest chemistry with water-soluble gold nanoparticle supraspheres. Nat. Nanotechnol. 12, 170–176 (2016).
Nishioka, Y., Yamaguchi, T., Yoshizawa, M. & Fujita, M. Unusual [2+4] and [2+2] cycloadditions of arenes in the confined cavity of self-assembled cages. J. Am. Chem. Soc. 129, 7000–7001 (2007).
Kang, J. & Rebek, J. Acceleration of a Diels–Alder reaction by a self-assembled molecular capsule. Nature 385, 50–52 (1997).
Taguchi, T., Isozaki, K. & Miki, K. Enhanced catalytic activity of self-assembled-monolayer-capped gold nanoparticles. Adv. Mater. 24, 6462–6467 (2012). Unique example in which silane alcoholysis is accelerated under confinement within a hydrophobic pocket formed between alkanethiol ligands on gold nanoparticles.
Sawano, T. et al. Metal–organic frameworks stabilize mono(phosphine)–metal complexes for broad-scope catalytic reactions. J. Am. Chem. Soc. 138, 9783–9786 (2016).
Roberts, J. M. et al. Urea metal–organic frameworks as effective and size-selective hydrogen-bond catalysts. J. Am. Chem. Soc. 134, 3334–3337 (2012).
Ikbal, S. A. et al. Bioinspired oxidation of methane in the confined spaces of molecular cages. Inorg. Chem. 58, 7220–7228 (2019).
Feng, X. et al. Metal-organic framework stabilizes a low-coordinate iridium complex for catalytic methane borylation. J. Am. Chem. Soc. 141, 11196–11203 (2019).
Zhang, D. et al. Tailored oxido-vanadium(V) cage complexes for selective sulfoxidation in confined spaces. Chem. Sci. 8, 789–794 (2017).
Chen, S. et al. A metal-organic cage incorporating multiple light harvesting and catalytic centres for photochemical hydrogen production. Nat. Commun. 7, 13169 (2016).
Gramage-Doria, R. et al. Gold(I) catalysis at extreme concentrations inside self-assembled nanospheres. Angew. Chem. Int. Ed. 53, 13380–13384 (2014).
Wu, S. et al. Supramolecular nanotubules as a catalytic regulator for palladium cations: Applications in selective catalysis. Angew. Chem. Int. Ed. 56, 11511–11514 (2017).
Kohyama, Y., Murase, T. & Fujita, M. Metal–organic proximity in a synthetic pocket. J. Am. Chem. Soc. 136, 2966–2969 (2014).
Pan, X. & Bao, X. The effects of confinement inside carbon nanotubes on catalysis. Acc. Chem. Res. 44, 553–562 (2011).
Xiao, J., Pan, X., Guo, S., Ren, P. & Bao, X. Toward fundamentals of confined catalysis in carbon nanotubes. J. Am. Chem. Soc. 137, 477–482 (2015).
Serp, P. & Castillejos, E. Catalysis in carbon nanotubes. ChemCatChem 2, 41–47 (2010).
Woese, C. The Genetic Code: The Molecular Basis for Genetic Expression (Harper & Row, 1967).
Fang, Y. et al. Bimolecular proximity of a ruthenium complex and methylene blue within an anionic porous coordination cage for enhancing photocatalytic activity. Chem. Sci. 10, 3529–3534 (2019).
Cullen, W., Misuraca, M. C., Hunter, C. A., Williams, N. H. & Ward, M. D. Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage. Nat. Chem. 8, 231–236 (2016).
Cullen, W. et al. Catalysis in a cationic coordination cage using a cavity-bound guest and surface-bound anions: Inhibition, activation, and autocatalysis. J. Am. Chem. Soc. 140, 2821–2828 (2018).
Wang, Q.-Q. et al. Self-assembled nanospheres with multiple endohedral binding sites pre-organize catalysts and substrates for highly efficient reactions. Nat. Chem. 8, 225–230 (2016).
Pordea, A. et al. Artificial metalloenzyme for enantioselective sulfoxidation based on vanadyl-loaded streptavidin. J. Am. Chem. Soc. 130, 8085–8088 (2008).
Chu, Z. et al. Supramolecular control of azobenzene switching on nanoparticles. J. Am. Chem. Soc. 141, 1949–1960 (2019).
Palma, A. et al. Cucurbit[7]uril as a supramolecular artificial enzyme for Diels–Alder reactions. Angew. Chem. Int. Ed. 129, 15894–15898 (2017).
Yu, Y. & Rebek, J. Reactions of folded molecules in water. Acc. Chem. Res. 51, 3031–3040 (2018).
Wang, K. et al. Electrostatic control of macrocyclization reactions within nanospaces. J. Am. Chem. Soc. 141, 6740–6747 (2019).
Mosca, S., Yu, Y., Gavette, J. V., Zhang, K.-D. & Rebek, J. A deep cavitand templates lactam formation in water. J. Am. Chem. Soc. 137, 14582–14585 (2015).
Shi, Q., Masseroni, D. & Rebek, J. Macrocyclization of folded diamines in cavitands. J. Am. Chem. Soc. 138, 10846–10848 (2016).
Wu, N.-W. & Rebek, J. Cavitands as chaperones for monofunctional and ring-forming reactions in water. J. Am. Chem. Soc. 138, 7512–7515 (2016).
Zhang, Q. & Tiefenbacher, K. Terpene cyclization catalysed inside a self-assembled cavity. Nat. Chem. 7, 197–202 (2015). Flexible terpene molecules are pre-organized into a folded state under confinement, facilitating the catalytic conversion of these linear substrates into cyclic terpenes.
Hooley, R. J. Taking on the turnover challenge. Nat. Chem. 8, 202–204 (2016).
Hooley, R. J. & Rebek, J. A deep cavitand catalyzes the Diels–Alder reaction of bound maleimides. Org. Biomol. Chem. 5, 3631–3636 (2007).
Yoshizawa, M., Tamura, M. & Fujita, M. Diels-Alder in aqueous molecular hosts: Unusual regioselectivity and efficient catalysis. Science 312, 251–254 (2006).
Pluth, M. D., Bergman, R. G. & Raymond, K. N. Acid catalysis in basic solution: A supramolecular host promotes orthoformate hydrolysis. Science 316, 85–88 (2007).
Fiedler, D., Bergman, R. G. & Raymond, K. N. Supramolecular catalysis of a unimolecular transformation: Aza-Cope rearrangement within a self-assembled host. Angew. Chem. Int. Ed. 43, 6748–6751 (2004).
Fiedler, D., van Halbeek, H., Bergman, R. G. & Raymond, K. N. Supramolecular catalysis of unimolecular rearrangements: Substrate scope and mechanistic insights. J. Am. Chem. Soc. 128, 10240–10252 (2006).
Hastings, C. J., Fiedler, D., Bergman, R. G. & Raymond, K. N. Aza Cope rearrangement of propargyl enammonium cations catalyzed by a self-assembled “nanozyme”. J. Am. Chem. Soc. 130, 10977–10983 (2008).
Dinca, L. E. et al. Unprecedented transformation of tetrathienoanthracene into pentacene on Ni(111). ACS Nano 7, 1652–1657 (2013).
de Oteyza, D. G. et al. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science 340, 1434–1437 (2013).
Vidal, D., Olivo, G. & Costas, M. Controlling selectivity in aliphatic C−H oxidation through supramolecular recognition. Chem. Eur. J. 24, 5042–5054 (2018).
Wang, C. et al. Product selectivity controlled by nanoporous environments in zeolite crystals enveloping rhodium nanoparticle catalysts for CO2 hydrogenation. J. Am. Chem. Soc. 141, 8482–8488 (2019).
Mochizuki, S., Kitao, T. & Uemura, T. Controlled polymerizations using metal–organic frameworks. Chem. Commun. 54, 11843–11856 (2018).
Tu, M. et al. Reversible optical writing and data storage in an anthracene-loaded metal–organic framework. Angew. Chem. Int. Ed. 58, 2423–2427 (2019). Confinement of four anthracene molecules within a single cavity leads to selective photodimerization of only two anthracene molecules because of steric restraints.
Yoshizawa, M., Takeyama, Y., Okano, T. & Fujita, M. Cavity-directed synthesis within a self-assembled coordination cage: Highly selective [2 + 2] cross-photodimerization of olefins. J. Am. Chem. Soc. 125, 3243–3247 (2003).
Ko, S. H. et al. Nanocage-confined synthesis of fluorescent polycyclic aromatic hydrocarbons in zeolite. J. Am. Chem. Soc. 140, 7101–7107 (2018).
Abedin, M. J., Liepold, L., Suci, P., Young, M. & Douglas, T. Synthesis of a cross-linked branched polymer network in the interior of a protein cage. J. Am. Chem. Soc. 131, 4346–4354 (2009).
Knossalla, J. et al. Shape-controlled nanoparticles in pore-confined space. J. Am. Chem. Soc. 140, 15684–15689 (2018).
Suzuki, K., Sato, S. & Fujita, M. Template synthesis of precisely monodisperse silica nanoparticles within self-assembled organometallic spheres. Nat. Chem. 2, 25–29 (2009).
Xu, Y.-T. et al. Cage-confinement pyrolysis route to ultrasmall tungsten carbide nanoparticles for efficient electrocatalytic hydrogen evolution. J. Am. Chem. Soc. 139, 5285–5288 (2017).
Meldrum, F. C., Wade, V. J., Nimmo, D. L., Heywood, B. R. & Mann, S. Synthesis of inorganic nanophase materials in supramolecular protein cages. Nature 349, 684–687 (1991).
Allen, M., Willits, D., Mosolf, J., Young, M. & Douglas, T. Protein cage constrained synthesis of ferrimagnetic iron oxide nanoparticles. Adv. Mater. 14, 1562–1565 (2002).
Douglas, T. et al. Protein engineering of a viral cage for constrained nanomaterials synthesis. Adv. Mater. 14, 415–418 (2002).
Kramer, R. M., Li, C., Carter, D. C., Stone, M. O. & Naik, R. R. Engineered protein cages for nanomaterial synthesis. J. Am. Chem. Soc. 126, 13282–13286 (2004).
Ramamurthy, V. & Turro, N. J. Photochemistry of organic molecules within zeolites: Role of cations. J. Incl. Phenom. Mol. Recognit. Chem. 21, 239–282 (1995).
Turro, N. J. & Wan, P. Photochemistry of phenyl alkyl ketones adsorbed on zeolite molecular sieves. Observation of pronounced effects on type I/type II photochemistry. Tetrahedron Lett. 25, 3655–3658 (1984).
Sivaguru, J. et al. Control of chirality by cations in confined spaces: Photooxidation of enecarbamates inside zeolite supercages. Photochem. Photobiol. 82, 123–131 (2006).
Turro, N. J., Cheng, C. C., Lei, X. G. & Flanigen, E. M. Size and selectivity of zeolite chemistry. A remarkable effect of additive on the products produced in the photolyses of ketones. J. Am. Chem. Soc. 107, 3739–3741 (1985).
Dusselier, M., Van Wouwe, P., Dewaele, A., Jacobs, P. A. & Sels, B. F. Shape-selective zeolite catalysis for bioplastics production. Science 349, 78–80 (2015). Shape-selectivity under confinement allows stereoselective conversion of l-lactic acid to (l,l)-lactide in near-quantitative yields, surpassing the industrial state-of-the-art.
Nakae, T. et al. Iron and ruthenium nanoparticles in carbon prepared by thermolysis of buckymetallocenes. Chem. Asian J. 4, 457–465 (2009).
Nakamura, E. et al. Electron microscopic imaging of a single group 8 metal atom catalyzing C–C bond reorganization of fullerenes. J. Am. Chem. Soc. 133, 14151–14153 (2011). Electron microscopy is used to monitor how confinement within carbon nanotubes facilitates the selective rearrangement of fullerenes, catalysed by Ru or Fe.
Koshino, M. et al. Analysis of the reactivity and selectivity of fullerene dimerization reactions at the atomic level. Nat. Chem. 2, 117–124 (2010).
Yoshizawa, M., Takeyama, Y., Kusukawa, T. & Fujita, M. Cavity-directed, highly stereoselective [2+2] photodimerization of olefins within self-assembled coordination cages. Angew. Chem. Int. Ed. 41, 1347–1349 (2002).
Han, Q. et al. Engineering chiral polyoxometalate hybrid metal–organic frameworks for asymmetric dihydroxylation of olefins. J. Am. Chem. Soc. 135, 10186–10189 (2013).
Gong, W. et al. Permanent porous hydrogen-bonded frameworks with two types of Brønsted acid sites for heterogeneous asymmetric catalysis. Nat. Commun. 10, 600 (2019).
Xu, H., Gao, J. & Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 7, 905–912 (2015).
Kim, M. et al. Creating favorable geometries for directing organic photoreactions in alkanethiolate monolayers. Science 331, 1312–1315 (2011).
Zheng, Y. B. et al. Surface-enhanced Raman spectroscopy to probe photoreaction pathways and kinetics of isolated reactants on surfaces: Flat versus curved substrates. Nano Lett. 12, 5362–5368 (2012).
Zdobinsky, T., Maiti, P. S. & Klajn, R. Support curvature and conformational freedom control chemical reactivity of immobilized species. J. Am. Chem. Soc. 136, 2711–2714 (2014).
Trinh, T. et al. DNA-imprinted polymer nanoparticles with monodispersity and prescribed DNA-strand patterns. Nat. Chem. 10, 184–192 (2017).
Zhou, T.-Y., Auer, B., Lee, S. J. & Telfer, S. G. Catalysts confined in programmed framework pores enable new transformations and tune reaction efficiency and selectivity. J. Am. Chem. Soc. 141, 1577–1582 (2019). Rational design of a MOF cavity that allows the reactivity and selectivity of aldol and Henry reactions to be tuned orthogonally.
Chen, X., Wang, Y., Wang, H., Kim, Y. & Lee, M. α-Helical peptide vesicles with chiral membranes as enantioselective nanoreactors. Chem. Commun. 53, 10958–10961 (2017).
Liu, S., Gan, H., Hermann, A. T., Rick, S. W. & Gibb, B. C. Kinetic resolution of constitutional isomers controlled by selective protection inside a supramolecular nanocapsule. Nat. Chem. 2, 847–852 (2010).
Yebeutchou, R. M. & Dalcanale, E. Highly selective monomethylation of primary amines through host−guest product sequestration. J. Am. Chem. Soc. 131, 2452–2453 (2009).
Masseroni, D., Mosca, S., Mower, M. P., Blackmond, D. G. & Rebek, J. Jr. Cavitands as reaction vessels and blocking groups for selective reactions in water. Angew. Chem. Int. Ed. 55, 8290–8293 (2016).
Fung, Y.-S., Yan, S.-C. & Wong, M.-K. Selective oxidation of unactivated C–H bonds by supramolecular control. Org. Biomol. Chem. 10, 3122–3130 (2012).
Chen, B., Holstein, J. J., Horiuchi, S., Hiller, W. G. & Clever, G. H. Pd(II) coordination sphere engineering: Pyridine cages, quinoline bowls, and heteroleptic pills binding one or two fullerenes. J. Am. Chem. Soc. 141, 8907–8913 (2019).
Tahara, K. et al. Self-assembled monolayers as templates for linearly nanopatterned covalent chemical functionalization of graphite and graphene surfaces. ACS Nano 12, 11520–11528 (2018).
Udayabhaskararao, T. et al. Tunable porous nanoallotropes prepared by post-assembly etching of binary nanoparticle superlattices. Science 358, 514–518 (2017).
Takezawa, H., Kanda, T., Nanjo, H. & Fujita, M. Site-selective functionalization of linear diterpenoids through U-shaped folding in a confined artificial cavity. J. Am. Chem. Soc. 141, 5112–5115 (2019).
Zhang, J. et al. A Pd@zeolite catalyst for nitroarene hydrogenation with high product selectivity by sterically controlled adsorption in the zeolite micropores. Angew. Chem. Int. Ed. 56, 9747–9751 (2017).
Ridelman, Y. et al. Metallic nanobowls by galvanic replacement reaction on heterodimeric nanoparticles. Small 8, 654–660 (2012).
Huang, W. & Ferris, J. P. One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis. J. Am. Chem. Soc. 128, 8914–8919 (2006).
Swadling, J. B., Coveney, P. V. & Greenwell, H. C. Clay minerals mediate folding and regioselective interactions of RNA: A large-scale atomistic simulation study. J. Am. Chem. Soc. 132, 13750–13764 (2010).
Turro, N. J. et al. Demonstration of a chemical transformation inside a fullerene. The reversible conversion of the allotropes of H2@C60. J. Am. Chem. Soc. 130, 10506–10507 (2008).
Micheroni, D., Lin, Z., Chen, Y.-S. & Lin, W. Luminescence enhancement of cis-[Ru(bpy)2(py)2]2+ via confinement within a metal–organic framework. Inorg. Chem. 58, 7645–7648 (2019).
Chatelet, B. et al. Superbases in confined space: Control of the basicity and reactivity of the proton transfer. J. Am. Chem. Soc. 135, 18659–18664 (2013).
Körner, S. K., Tucci, F. C., Rudkevich, D. M., Heinz, T. & Rebek, J. A self-assembled cylindrical capsule: New supramolecular phenomena through encapsulation. Chem. Eur. J. 6, 187–195 (2000).
Chen, L.-J. et al. Construction of porphyrin-containing metallacycle with improved stability and activity within mesoporous carbon. J. Am. Chem. Soc. 140, 5049–5052 (2018).
Kang, Y.-H. et al. Fabrication of isolated metal–organic polyhedra in confined cavities: Adsorbents/catalysts with unusual dispersity and activity. J. Am. Chem. Soc. 138, 6099–6102 (2016).
Tang, T. & Hou, Y. Chemical confinement and utility of lithium polysulfides in lithium sulfur batteries. Small Methods https://doi.org/10.1002/smtd.201900001 (2019).
Ren, W., Ma, W., Zhang, S. & Tang, B. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 23, 707–732 (2019).
Liu, W., Lin, D., Pei, A. & Cui, Y. Stabilizing lithium metal anodes by uniform Li-ion flux distribution in nanochannel confinement. J. Am. Chem. Soc. 138, 15443–15450 (2016).
Hertzberg, B., Alexeev, A. & Yushin, G. Deformations in Si−Li anodes upon electrochemical alloying in nano-confined space. J. Am. Chem. Soc. 132, 8548–8549 (2010).
Zhang, J., Hu, H., Li, Z. & Lou, X. W. Double-shelled nanocages with cobalt hydroxide inner shell and layered double hydroxides outer shell as high-efficiency polysulfide mediator for lithium–sulfur batteries. Angew. Chem. Int. Ed. 55, 3982–3986 (2016).
Xin, S. et al. Smaller sulfur molecules promise better lithium–sulfur batteries. J. Am. Chem. Soc. 134, 18510–18513 (2012).
Sachtler, W. M. H. Metal clusters in zeolites: An intriguing class of catalysts. Acc. Chem. Res. 26, 383–387 (1993).
Liu, L. et al. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 16, 132–138 (2017).
Sun, Q. et al. Subnanometric hybrid Pd-M(OH)2, M = Ni, Co, clusters in zeolites as highly efficient nanocatalysts for hydrogen generation. Chem 3, 477–493 (2017).
Mon, M. et al. Stabilized Ru[(H2O)6]3+ in confined spaces (MOFs and zeolites) catalyzes the imination of primary alcohols under atmospheric conditions with wide scope. ACS Catal. 8, 10401–10406 (2018).
Sawada, T., Yoshizawa, M., Sato, S. & Fujita, M. Minimal nucleotide duplex formation in water through enclathration in self-assembled hosts. Nat. Chem. 1, 53–56 (2009). An unstable assembly composed of a single DNA base pair is stabilized under confinement within a coordination cage.
Cao, H. & De Feyter, S. Amplification of chirality in surface-confined supramolecular bilayers. Nat. Commun. 9, 3416 (2018).
Hazen, R. M. & Sholl, D. S. Chiral selection on inorganic crystalline surfaces. Nat. Mater. 2, 367–374 (2003).
Li, H. et al. Mechanical bond-induced radical stabilization. J. Am. Chem. Soc. 135, 456–467 (2013).
Howlader, P., Mondal, B., Purba, P. C., Zangrando, E. & Mukherjee, P. S. Self-assembled Pd(II) barrels as containers for transient merocyanine form and reverse thermochromism of spiropyran. J. Am. Chem. Soc. 140, 7952–7960 (2018).
Takezawa, H., Akiba, S., Murase, T. & Fujita, M. Cavity-directed chromism of phthalein dyes. J. Am. Chem. Soc. 137, 7043–7046 (2015).
Samanta, D. et al. Reversible chromism of spiropyran in the cavity of a flexible coordination cage. Nat. Commun. 9, 641 (2018).
Rupar, P. A., Staroverov, V. N. & Baines, K. M. A cryptand-encapsulated germanium(II) dication. Science 322, 1360–1363 (2008).
Iwasawa, T., Hooley, R. J. & Rebek, J. Stabilization of labile carbonyl addition intermediates by a synthetic receptor. Science 317, 493–496 (2007).
Rizzuto, F. J., Ramsay, W. J. & Nitschke, J. R. Otherwise unstable structures self-assemble in the cavities of cuboctahedral coordination cages. J. Am. Chem. Soc. 140, 11502–11509 (2018). A highly unstable penta-coordinated Cd II complex is stabilized under confinement via a combination of intermolecular interactions and steric constraint within the cavity of a coordination cage.
Cram, D. J., Tanner, M. E. & Thomas, R. The taming of cyclobutadiene. Angew. Chem. Int. Ed. 30, 1024–1027 (1991).
Liu, A., Traulsen, C. H.-H. & Cornelissen, J. J. L. M. Nitroarene reduction by a virus protein cage based nanoreactor. ACS Catal. 6, 3084–3091 (2016).
Yamashina, M., Sei, Y., Akita, M. & Yoshizawa, M. Safe storage of radical initiators within a polyaromatic nanocapsule. Nat. Commun. 5, 4662 (2014).
Mal, P., Breiner, B., Rissanen, K. & Nitschke, J. R. White phosphorus is air-stable within a self-assembled tetrahedral capsule. Science 324, 1697–1699 (2009).
Kaphan, D. M., Levin, M. D., Bergman, R. G., Raymond, K. N. & Toste, F. D. A supramolecular microenvironment strategy for transition metal catalysis. Science 350, 1235–1238 (2015).
Pluth, M. D., Bergman, R. G. & Raymond, K. N. Supramolecular catalysis of orthoformate hydrolysis in basic solution: An enzyme-like mechanism. J. Am. Chem. Soc. 130, 11423–11429 (2008).
Fan, Q. et al. Precise monoselective aromatic C–H bond activation by chemisorption of meta-aryne on a metal surface. J. Am. Chem. Soc. 140, 7526–7532 (2018).
Warmuth, R., Kerdelhué, J.-L., Sánchez Carrera, S., Langenwalter, K. J. & Brown, N. Rate acceleration through dispersion interactions: Effect of a hemicarcerand on the transition state of inner phase decompositions of diazirines. Angew. Chem. Int. Ed. 41, 96–99 (2002).
Juríček, M. et al. Induced-fit catalysis of corannulene bowl-to-bowl inversion. Nat. Chem. 6, 222–228 (2014). An important example that quantifies the stabilization of the transition state associated with corannulene bowl-to-bowl inversion under confinement.
Lee, T.-C. et al. Chemistry inside molecular containers in the gas phase. Nat. Chem. 5, 376–382 (2013).
Warmuth, R. o-Benzyne: Strained alkyne or cumulene?—NMR characterization in a molecular container. Angew. Chem. Int. Ed. 36, 1347–1350 (1997).
Yoshizawa, M., Kusukawa, T., Fujita, M. & Yamaguchi, K. Ship-in-a-bottle synthesis of otherwise labile cyclic trimers of siloxanes in a self-assembled coordination cage. J. Am. Chem. Soc. 122, 6311–6312 (2000).
Nuñez-Lopez, A. et al. Direct visualization of pyrrole reactivity by confined oxidation in a cyclodextrin metal–organic framework. Angew. Chem. Int. Ed. 58, 9179–9183 (2019).
Yumura, T., Takeuchi, M., Kobayashi, H. & Kuroda, Y. Effects of ZSM-5 zeolite confinement on reaction intermediates during dioxygen activation by enclosed dicopper cations. Inorg. Chem. 48, 508–517 (2009).
Woertink, J. S. et al. A [Cu2O]2+ core in Cu-ZSM-5, the active site in the oxidation of methane to methanol. Proc. Natl Acad. Sci. USA 106, 18908–18913 (2009).
Sushkevich, V. L., Palagin, D., Ranocchiari, M. & van Bokhoven, J. A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356, 523–527 (2017).
Bälter, M. et al. Emission color tuning and white-light generation based on photochromic control of energy transfer reactions in polymer micelles. Chem. Sci. 7, 5867–5871 (2016).
Zhang, H. et al. Zeolite-confined carbon dots: Tuning thermally activated delayed fluorescence emission via energy transfer. Mater. Chem. Front. https://doi.org/10.1039/C9QM00549H (2020).
Naren, G. et al. An all-photonic full color RGB system based on molecular photoswitches. Nat. Commun. 10, 3996 (2019). Confinement of blue fluorescent donor molecules with red and green photochromic acceptor molecules within micelles allows energy transfer between the confined species, resulting in controlled emission throughout the entire RGB colour system.
Liu, J. et al. Carbon dots in zeolites: A new class of thermally activated delayed fluorescence materials with ultralong lifetimes. Sci. Adv. 3, e1603171 (2017).
Zhang, Q.-W. et al. Multicolor photoluminescence including white-light emission by a single host–guest complex. J. Am. Chem. Soc. 138, 13541–13550 (2016).
Ams, M. R., Ajami, D., Craig, S. L., Yang, J.-S. & Rebek, J. “Too small, too big, and just right” − Optical sensing of molecular conformations in self-assembled capsules. J. Am. Chem. Soc. 131, 13190–13191 (2009).
Hua, B. et al. Supramolecular solid-state microlaser constructed from pillar[5]arene-based host–guest complex microcrystals. J. Am. Chem. Soc. 140, 15651–15654 (2018).
Mako, T. L., Racicot, J. M. & Levine, M. Supramolecular luminescent sensors. Chem. Rev. 119, 322–477 (2019).
Jiang, Y. et al. Maximizing photoresponsive efficiency by isolating metal–organic polyhedra into confined nanoscaled spaces. J. Am. Chem. Soc. 141, 8221–8227 (2019).
Biedermann, F., Elmalem, E., Ghosh, I., Nau, W. M. & Scherman, O. A. Strongly fluorescent, switchable perylene bis(diimide) host–guest complexes with cucurbit[8]uril in water. Angew. Chem. Int. Ed. 51, 7739–7743 (2012).
Song, N., Kakuta, T., Yamagishi, T., Yang, Y.-W. & Ogoshi, T. Molecular-scale porous materials based on pillar[n]arenes. Chem 4, 2029–2053 (2018).
Xue, M., Yang, Y., Chi, X., Zhang, Z. & Huang, F. Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 45, 1294–1308 (2012).
Ogoshi, T., Yamagishi, T. & Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar[n]arenes: New key players for supramolecular chemistry. Chem. Rev. 116, 7937–8002 (2016).
Tian, Y., Yan, X., Saha, M. L., Niu, Z. & Stang, P. J. Hierarchical self-assembly of responsive organoplatinum(II) metallacycle–TMV complexes with turn-on fluorescence. J. Am. Chem. Soc. 138, 12033–12036 (2016).
Jiang, T., Wang, X., Wang, J., Hu, G. & Ma, X. Humidity- and temperature-tunable multicolor luminescence of cucurbit[8]uril-based supramolecular assembly. ACS Appl. Mater. Interfaces 11, 14399–14407 (2019).
Kundu, P. K., Olsen, G. L., Kiss, V. & Klajn, R. Nanoporous frameworks exhibiting multiple stimuli responsiveness. Nat. Commun. 5, 3588 (2014).
Rizzuto, F. J., von Krbek, L. K. S. & Nitschke, J. R. Strategies for binding multiple guests in metal–organic cages. Nat. Rev. Chem. 3, 204–222 (2019).
Biedermann, F., Hathazi, D. & Nau, W. M. Associative chemosensing by fluorescent macrocycle–dye complexes – A versatile enzyme assay platform beyond indicator displacement. Chem. Commun. 51, 4977–4980 (2015).
Sinn, S. & Biedermann, F. Chemical sensors based on cucurbit[n]uril macrocycles. Isr. J. Chem. 58, 357–412 (2018).
Wu, G., Olesińska, M., Wu, Y., Matak-Vinkovic, D. & Scherman, O. A. Mining 2:2 complexes from 1:1 stoichiometry: Formation of cucurbit[8]uril–diarylviologen quaternary complexes favored by electron-donating substituents. J. Am. Chem. Soc. 139, 3202–3208 (2017).
Yamashina, M. et al. Preparation of highly fluorescent host–guest complexes with tunable color upon encapsulation. J. Am. Chem. Soc. 137, 9266–9269 (2015). Excellent example illustrating how co-encapsulation of fluorescent dyes with other species can be employed to tune the emission colour of the dye.
Jana, A. et al. Functionalised tetrathiafulvalene- (TTF-) macrocycles: Recent trends in applied supramolecular chemistry. Chem. Soc. Rev. 47, 5614–5645 (2018).
Klosterman, J. K., Yamauchi, Y. & Fujita, M. Engineering discrete stacks of aromatic molecules. Chem. Soc. Rev. 38, 1714–1725 (2009).
Rizzuto, F. J., Wood, D. M., Ronson, T. K. & Nitschke, J. R. Tuning the redox properties of fullerene clusters within a metal–organic capsule. J. Am. Chem. Soc. 139, 11008–11011 (2017).
Matsumoto, K. et al. A peanut-shaped polyaromatic capsule: Solvent-dependent transformation and electronic properties of a non-contacted fullerene dimer. Angew. Chem. Int. Ed. 58, 8463–8467 (2019).
Nurttila, S. S., Becker, R., Hessels, J., Woutersen, S. & Reek, J. N. H. Photocatalytic hydrogen evolution by a synthetic [FeFe] hydrogenase mimic encapsulated in a porphyrin cage. Chem. Eur. J. 24, 16395–16406 (2018).
Kung, C.-W. et al. Increased electrical conductivity in a mesoporous metal–organic framework featuring metallacarboranes guests. J. Am. Chem. Soc. 140, 3871–3875 (2018).
Barnes, J. C. et al. Semiconducting single crystals comprising segregated arrays of complexes of C60. J. Am. Chem. Soc. 137, 2392–2399 (2015).
Aubrey, M. L. et al. Chemiresistive detection of gaseous hydrocarbons and interrogation of charge transport in Cu[Ni(2,3-pyrazinedithiolate)2] by gas adsorption. J. Am. Chem. Soc. 141, 5005–5013 (2019).
Fahrenbach, A. C. et al. A semiconducting organic radical cationic host–guest complex. ACS Nano 6, 9964–9971 (2012).
Talin, A. A. et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science 343, 66–69 (2014). Unique example in which charge transfer between a confined species and the metal–organic framework of its environment results in conductivity throughout the material.
Huang, Q.-Q. et al. Tunable electrical conductivity of a new 3D MOFs: Cu-TATAB. Inorg. Chem. Commun. 105, 119–124 (2019).
Schneider, C. et al. High electrical conductivity and high porosity in a guest@MOF material: Evidence of TCNQ ordering within Cu3BTC2 micropores.Chem. Sci. 9, 7405–7412 (2018).
Küchler, A., Yoshimoto, M., Luginbühl, S., Mavelli, F. & Walde, P. Enzymatic reactions in confined environments. Nat. Nanotechnol. 11, 409–420 (2016).
Warshel, A. et al. Electrostatic basis for enzyme catalysis. Chem. Rev. 106, 3210–3235 (2006).
Gao, J. et al. Mechanisms and free energies of enzymatic reactions. Chem. Rev. 106, 3188–3209 (2006).
Fracaroli, A. M. et al. Seven post-synthetic covalent reactions in tandem leading to enzyme-like complexity within metal–organic framework crystals. J. Am. Chem. Soc. 138, 8352–8355 (2016).
Wei, H. & Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 42, 6060–6093 (2013).
Meyer, H.-P. et al. The use of enzymes in organic synthesis and the life sciences: Perspectives from the Swiss Industrial Biocatalysis Consortium (SIBC). Catal. Sci. Technol. 3, 29–40 (2013).
Future Perspectives in Catalysis (Dutch National Research School Combination, 2009).
Acknowledgements
This work was supported by the European Research Council (grant no. 336080). A.B.G. acknowledges funding from the Mortimer B. Zuckerman STEM Leadership Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grommet, A.B., Feller, M. & Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 15, 256–271 (2020). https://doi.org/10.1038/s41565-020-0652-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0652-2
This article is cited by
-
Electron density-based GPT for optimization and suggestion of host–guest binders
Nature Computational Science (2024)
-
Artificial molecular pumps
Nature Reviews Methods Primers (2024)
-
Crystallization of molecular layers produced under confinement onto a surface
Nature Communications (2024)
-
Chiral BINOL-phosphate assembled single hexagonal nanotube in aqueous solution for confined rearrangement acceleration
Nature Communications (2024)
-
Elastocapillarity-driven 2D nano-switches enable zeptoliter-scale liquid encapsulation
Nature Communications (2024)