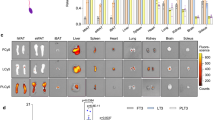
Abstract
Photodynamic therapy and adipose browning induction are two promising approaches to reverse obesity. The former strategy acts rapidly and locally, whereas the latter has a more gradual and widespread effect. Despite their complementarity, they have rarely been combined and imaged non-invasively in vivo. Here we introduce an adipose-targeting hepatitis B core protein complex that contains a traceable photosensitizer (ZnPcS4 (zinc phthalocyanine tetrasulfonate)) and a browning agent (rosiglitazone) that allows simultaneous photodynamic and browning treatments, with photoacoustic molecular imaging. After intravenous injection in obese mice, the complex binds specifically to white adipose tissues, especially those rich in blood supply, and drives adipose reduction thanks to the synergy of ZnPcS4 photodynamics and rosiglitazone browning. Using photoacoustic molecular imaging, we could monitor the changes induced by the treatment, which included complex activity, lipid catabolism and angiogenesis. Our findings demonstrate the anti-obesity potential of our feedback-based synergic regimen orchestrated by the targeted hepatitis B core complex.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All the datasets in the current study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Friedman, J. M. Causes and control of excess body fat. Nature 459, 340–342 (2009).
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
Afshin, A. et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377, 13–27 (2017).
Van Gaal, L. F., Mertens, I. L. & De Block, C. E. Mechanisms linking obesity with cardiovascular disease. Nature 444, 875–880 (2006).
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
Olson, O. C., Quail, D. F. & Joyce, J. A. Obesity and the tumor microenvironment. Science 358, 1130–1131 (2017).
Heymsfield, S. B. & Wadden, T. A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 376, 254–266 (2017).
Vetter, M. L., Faulconbridge, L. F., Webb, V. L. & Wadden, T. A. Behavioral and pharmacologic therapies for obesity. Nat. Rev. Endocrinol. 6, 578–588 (2010).
Agostinis, P. et al. Photodynamic therapy of cancer: an update. Ca. Cancer J. Clin. 61, 250–281 (2011).
Zhou, Z., Song, J., Nie, L. & Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 45, 6597–6626 (2016).
Chilakamarthi, U. & Giribabu, L. Photodynamic therapy: past, present and future. Chem. Rec. 17, 775–802 (2017).
Wanner, M. et al. Use of photodynamic therapy and sterile water to target adipose tissue. Dermatologic Surg. 41, 803–811 (2015).
Peirce, V., Carobbio, S. & Vidal-Puig, A. The different shades of fat. Nature 510, 76–83 (2014).
Liu, Y., Nie, L. & Chen, X. Photoacoustic molecular imaging: from multiscale biomedical applications towards early-stage theranostics. Trends Biotechnol. 34, 420–433 (2016).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Wang, L. V. & Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 13, 627–638 (2016).
Valluru, K. S., Wilson, K. E. & Willmann, J. K. Photoacoustic imaging in oncology: translational preclinical and early clinical experience. Radiology 280, 332–349 (2016).
Reber, J. et al. Non-invasive measurement of brown fat metabolism based on optoacoustic imaging of hemoglobin gradients. Cell Metab. 27, 689–701 (2018).
Lovell, J. F., Liu, T. W. B., Chen, J. & Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 110, 2839–2857 (2010).
Calixto, G. M. F. et al. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: a review. Molecules 21, 342 (2016).
Ho, C. J. H. et al. Multifunctional photosensitizer-based contrast agents for photoacoustic imaging. Sci. Rep. 4, 5342 (2014).
Bartelt, A. & Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10, 24–36 (2014).
Mannerås-Holm, L. & Krook, A. Targeting adipose tissue angiogenesis to enhance insulin sensitivity. Diabetologia 55, 2562–2564 (2012).
Festuccia, W. T., Laplante, M., Berthiaume, M., Gélinas, Y. & Deshaies, Y. PPARγ agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia 49, 2427–2436 (2006).
Clarke, B. E. et al. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature 330, 381–384 (1987).
Ji, M. et al. Hepatitis B core VLP-based mis-disordered tau vaccine elicits strong immune response and alleviates cognitive deficits and neuropathology progression in Tau.P301S mouse model of Alzheimer’s disease and frontotemporal dementia. Alzheimers Res. Ther. 10, 55 (2018).
Lu, Y. et al. Assessing sequence plasticity of a virus-like nanoparticle by evolution toward a versatile scaffold for vaccines and drug delivery. Proc. Natl Acad. Sci. USA 112, 12360–12365 (2015).
Kolonin, M. G., Saha, P. K., Chan, L., Pasqualini, R. & Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 10, 625–632 (2004).
Won, Y. W. et al. Oligopeptide complex for targeted non-viral gene delivery to adipocytes. Nat. Mater. 13, 1157–1164 (2014).
Shan, W. et al. Improved stable indocyanine green (ICG)-mediated cancer optotheranostics with naturalized hepatitis B core particles. Adv. Mater. 30, 1707567 (2018).
Kershaw, E. E. et al. PPARγ regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 293, 1736–1745 (2007).
Tang, J. et al. Obesity-associated family with sequence similarity 13, member A (FAM13A) is dispensable for adipose development and insulin sensitivity. Int. J. Obes. 43, 1269–1280 (2019).
Thiam, A. R., Farese, R. V. J. & Walther, T. C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 14, 775–786 (2013).
Zhang, C. et al. A cystine knot peptide targeting integrin αvβ6 for photoacoustic and fluorescence imaging of tumors in living subjects. J. Nucl. Med. 57, 1629–1634 (2016).
Crandall, D. L., Goldstein, B. M., Huggins, F. & Cervoni, P. Adipocyte blood flow: influence of age, anatomic location, and dietary manipulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 247, R46–R51 (1984).
Wang, P. et al. Absence of an adipogenic effect of rosiglitazone on mature 3T3-L1 adipocytes: increase of lipid catabolism and reduction of adipokine expression. Diabetologia 50, 654–665 (2007).
Li, G., Gong, J., Lei, H., Liu, J. & Xu, X. Z. S. Promotion of behavior and neuronal function by reactive oxygen species in C. elegans. Nat. Commun. 7, 13234 (2016).
Zou, Z., Chang, H., Li, H. & Wang, S. Induction of reactive oxygen species: an emerging approach for cancer therapy. Apoptosis 22, 1321–1335 (2017).
Yaribeygi, H., Farrokhi, F. R., Butler, A. E. & Sahebkar, A. Insulin resistance: review of the underlying molecular mechanisms. J. Cell Physiol. 234, 8152–8161 (2019).
Krawczyk, S. A., Haller, J. F., Ferrante, T., Zoeller, R. A. & Corkey, B. E. Reactive oxygen species facilitate translocation of hormone sensitive lipase to the lipid droplet during lipolysis in human differentiated adipocytes. PLoS ONE 7, e34904 (2012).
Straus, D. S. & Glass, C. K. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28, 551–558 (2007).
Bensinger, S. J. & Tontonoz, P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 454, 470–477 (2008).
Polvani, S., Tarocchi, M. & Galli, A. PPAR and oxidative stress: Con(β) catenating NRF2 and FOXO. PPAR Res 2012, 641087 (2012).
Xue, Y. et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 9, 99–109 (2009).
Reuter, S., Gupta, S. C., Chaturvedi, M. M. & Aggarwal, B. B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49, 1603–1616 (2010).
Sackmann-Sala, L., Berryman, D. E., Munn, R. D., Lubbers, E. R. & Kopchick, J. J. Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity 20, 101–111 (2012).
Nathan, D. M. Rosiglitazone and cardiotoxicity—weighing the evidence. N. Engl. J. Med. 357, 64–66 (2007).
Ratziu, V. et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 51, 445–453 (2010).
Moreno, A. M. et al. Immune-orthogonal orthologues of AAV capsids and of Cas9 circumvent the immune response to the administration of gene therapy. Nat. Biomed. Eng. 3, 806–816 (2019).
Wu, Z. et al. In vivo dual-scale photoacoustic surveillance and assessment of burn healing. Biomed. Opt. Express 10, 3425–3433 (2019).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (U190420008 and 81922034&91859113) and the Science Fund for Distinguished Young Scholars of Fujian Province (2018J06024). We thank X. Wang for her technical support in immunological experiments, Z. Wu for his guidance in PA data processing and S. Li for his help in PA data collection.
Author information
Authors and Affiliations
Contributions
R.C. and L.N. conceived and designed the study. R.C., S.H., T.L., H.M., W.S. and J.L. carried out the experiments. R.C., S.H., F.D. and J.Z. analysed the data. R.C. wrote the first draft of the manuscript. L.R. and L.N. edited the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Frank Sainsbury and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Competition assays.
a,b, Representative confocal microscope images of adipose MECs (a) and mature adipocytes (b) pretreated with/without Pat-HBc for 2 h and further incubated with HBc/RSG&ZnPcS4 or Pat-HBc/RSG&ZnPcS4 for 12 h. Prohibitin was stained with phycoerythrin-conjugated anti-prohibitin antibody (magenta channel). Nuclei were counterstained with DAPI (blue channel). The experiments were repeated three times with similar results. Scale bars, 20 μm.
Extended Data Fig. 2 Safety assessments.
a, Representative H&E stained images of major organs including liver, kidney, heart, lung and spleen in different groups after two treatment cycles. The experiment was repeated three times with similar results. Scale bars, 50 μm. b-j, Serum biochemistry in obese mice after different treatments (n = 7 mice). Serum TP (b), ALT (c) and AST (d) reflect hepatic function. Serum uric acid (e), urea (f) and creatinine (g) reflect renal function. Serum CK (h), CK-MB (i) and LDH (j) reflect cardiac function. Asterisks in c and d denote significant differences compared with the control, RSG, ZnPcS4, RSG&ZnPcS4 and Pat-HBc/ZnPcS4 groups, p < 0.05. k-m, Serum IgG (k), IL-4 (l) and IFN-γ (m) levels indicative of cellular and humoral immune responses in obese mice at designated intervals after tail-vein injection with PBS, OVA, HBc or Pat-HBc (n = 6 mice). Time points for the first injection and the second injection were set on day 0 and day 7, respectively. Asterisks denote a significant difference from the PBS group, p < 0.05. Hash signs denote significant differences compared with the OVA and HBc groups, p < 0.05. All statistical data are expressed as mean ± s.d. Statistical significance was assessed via a one-way ANOVA with Duncan post-hoc test.
Supplementary information
Supplementary Information
Supplementary Figs. 1–27 and Tables 1–4.
Supplementary Video 1
3D laser-scanning confocal microscopy for analyzing penetration properties of different ZnPcS4 formulations in MEC spheroids (scan depth: ~240 μm).
Supplementary Video 2
3D PA display of abdominal adipose vessels in the obese mouse of Pat-HBc/RSG&ZnPcS4 group before and after treatment.
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data and Unprocessed Blots
Source Data Extended Data Figures
Statistical Source Data
Rights and permissions
About this article
Cite this article
Chen, R., Huang, S., Lin, T. et al. Photoacoustic molecular imaging-escorted adipose photodynamic–browning synergy for fighting obesity with virus-like complexes. Nat. Nanotechnol. 16, 455–465 (2021). https://doi.org/10.1038/s41565-020-00844-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-00844-6
This article is cited by
-
Exosome-based delivery strategies for tumor therapy: an update on modification, loading, and clinical application
Journal of Nanobiotechnology (2024)
-
Self-assembled peptide-dye nanostructures for in vivo tumor imaging and photodynamic toxicity
npj Imaging (2024)
-
A rationally designed nuclei-targeting FAPI 04-based molecular probe with enhanced tumor uptake for PET/CT and fluorescence imaging
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
A biodegradable, flexible photonic patch for in vivo phototherapy
Nature Communications (2023)
-
Cucurbit[8]uril-based water-dispersible assemblies with enhanced optoacoustic performance for multispectral optoacoustic imaging
Nature Communications (2023)