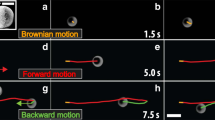
Abstract
Active matters are out-of-equilibrium systems that convert energy from the environment to mechanical motion. Non-reciprocal interaction between active matters may lead to collective intelligence beyond the capability of individuals. In nature, such emergent behaviours are ubiquitously observed in animal colonies, giving these species remarkable adaptive capability. In artificial systems, however, the emergence of non-trivial collective intelligent dynamics remains undiscovered. Here we show that a simple ion-exchange reaction can couple self-propelled ZnO nanorods and sulfonated polystyrene microbeads together. Chemical communication is established that enhances the reactivity and motion of both nanorods and the microbeads, resulting in the formation of an active swarm of nanorod–microbead complexes. We demonstrate that the swarm is capable of macroscopic phase segregation and intelligent consensus decision-making.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
West, S. A., Griffin, A. S., Gardner, A. & Diggle, S. P. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 4, 597–607 (2006).
Reynolds, C. W. Flocks, Herds and Schools: A Distributed Behavioral Model Vol. 21 (ACM, 1987).
Bialek, W. et al. Statistical mechanics for natural flocks of birds. Proc. Natl Acad. Sci. USA 109, 4786–4791 (2012).
Nelson, B. J., Kaliakatsos, I. K. & Abbott, J. J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 12, 55–85 (2010).
Wang, J., Xiong, Z., Zheng, J., Zhan, X. & Tang, J. Light-driven micro/nanomotor for promising biomedical tools: principle, challenge, and prospect. Acc. Chem. Res. 51, 1957–1965 (2018).
Wang, J. Nanomachines: Fundamentals and Applications (John Wiley & Sons, 2013).
Singh, D. P., Choudhury, U., Fischer, P. & Mark, A. G. Non-equilibrium assembly of light-activated colloidal mixtures. Adv. Mater. 29, https://doi.org/10.1002/adma.201701328 (2017).
Bechinger, C. et al. Active particles in complex and crowded environments. Rev. Mod. Phys. 88, 045006 (2016).
Niu, R. & Palberg, T. Modular approach to microswimming. Soft. Matter 14, 7554–7568 (2018).
Miskin, M. Z. et al. Electronically integrated, mass-manufactured, microscopic robots. Nature 584, 557–561 (2020).
Handl, J. & Meyer, B. Ant-based and swarm-based clustering. Swarm. Intell. 1, 95–113 (2007).
Coyte, K. Z., Schluter, J. & Foster, K. R. The ecology of the microbiome: networks, competition, and stability. Science 350, 663–666 (2015).
Faust, K. & Raes, J. Microbial interactions: from networks to models. Nat. Rev. Microbiol. 10, 538–550 (2012).
Mukherjee, S. & Bassler, B. L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 17, 371–382 (2019).
Dag, S., Wang, S. & Wang, L. W. Large surface dipole moments in ZnO nanorods. Nano Lett. 11, 2348–2352 (2011).
Zhou, J., Xu, N. S. & Wang, Z. L. Dissolving behavior and stability of ZnO wires in biofluids: a study on biodegradability and biocompatibility of ZnO nanostructures. Adv. Mater. 18, 2432–2435 (2006).
Valtiner, M., Borodin, S. & Grundmeier, G. Stabilization and acidic dissolution mechanism of single-crystalline ZnO (0001) surfaces in electrolytes studied by in-situ AFM imaging and ex-situ LEED. Langmuir 24, 5350–5358 (2008).
Dey, K. K., Bhandari, S., Bandyopadhyay, D., Basu, S. & Chattopadhyay, A. The pH taxis of an intelligent catalytic microbot. Small 9, 1916–1920 (2013).
Tu, Y., Peng, F. & Wilson, D. A. Motion manipulation of micro- and nanomotors. Adv. Mater. 29, https://doi.org/10.1002/adma.201701970 (2017).
Niu, R., Palberg, T. & Speck, T. Self-assembly of colloidal molecules due to self-generated flow. Phys. Rev. Lett. 119, 028001 (2017).
Niu, R., Fischer, A., Palberg, T. & Speck, T. Dynamics of binary active clusters driven by ion-exchange particles. ACS Nano. 12, 10932–10938 (2018).
Soto, R. & Golestanian, R. Self-assembly of catalytically active colloidal molecules: tailoring activity through surface chemistry. Phys. Rev. Lett. 112, 068301 (2014).
Bricard, A., Caussin, J. B., Desreumaux, N., Dauchot, O. & Bartolo, D. Emergence of macroscopic directed motion in populations of motile colloids. Nature 503, 95–98 (2013).
Vicsek, T., Czirok, A., Ben-Jacob, E., Cohen, I. I. & Shochet, O. Novel type of phase transition in a system of self-driven particles. Phys. Rev. Lett. 75, 1226–1229 (1995).
Duan, W., Liu, R. & Sen, A. Transition between collective behaviors of micromotors in response to different stimuli. J. Am. Chem. Soc. 135, 1280–1283 (2013).
Altemose, A. et al. Chemically controlled spatiotemporal oscillations of colloidal assemblies. Angew. Chem. Int. Ed. 56, 7817–7821 (2017).
Singh, D. P. et al. Interface-mediated spontaneous symmetry breaking and mutual communication between drops containing chemically active particles. Nat. Commun. 11, 2210 (2020).
Kudrolli, A., Lumay, G., Volfson, D. & Tsimring, L. S. Swarming and swirling in self-propelled polar granular rods. Phys. Rev. Lett. 100, 058001 (2008).
Wensink, H. & Löwen, H. Aggregation of self-propelled colloidal rods near confining walls. Phys. Rev. E. 78, 031409 (2008).
Bricard, A. et al. Emergent vortices in populations of colloidal rollers. Nat. Commun. 6, 7470 (2015).
Narayan, V., Ramaswamy, S. & Menon, N. Long-lived giant number fluctuations in a swarming granular nematic. Science 317, 105–108 (2007).
Whiteley, M., Diggle, S. P. & Greenberg, E. P. Progress in and promise of bacterial quorum sensing research. Nature 551, 313–320 (2017).
Peng, C., Turiv, T., Guo, Y., Wei, Q.-H. & Lavrentovich, O. D. Command of active matter by topological defects and patterns. Science 354, 882–885 (2016).
Park, S. et al. Motion to form a quorum. Science 301, 188 (2003).
Bauerle, T., Fischer, A., Speck, T. & Bechinger, C. Self-organization of active particles by quorum sensing rules. Nat. Commun. 9, 3232 (2018).
Solon, A. P. et al. Pressure is not a state function for generic active fluids. Nat. Phys. 11, 673–678 (2015).
Pratt, S. C., Mallon, E. B., Sumpter, D. J. & Franks, N. R. Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 52, 117–127 (2002).
Visscher, P. K. & Camazine, S. Collective decisions and cognition in bees. Nature 397, 400–400 (1999).
Needleman, D. & Dogic, Z. Active matter at the interface between materials science and cell biology. Nat. Rev. Mater. 2, 17048 (2017).
Greene, L. E. et al. Low-temperature wafer-scale production of ZnO nanowire arrays. Angew. Chem. Int. Ed. 42, 3031–3034 (2003).
Wang, Y. et al. Synthetic strategies toward DNA-coated colloids that crystallize. J. Am. Chem. Soc. 137, 10760–10766 (2015).
Qi, G. et al. Facile and scalable synthesis of monodispersed spherical capsules with a mesoporous shell. Chem. Mater. 22, 2693–2695 (2010).
Gibson, H. W. & Bailey, F. C. Chemical modification of polymers. 13. Sulfonation of polystyrene surfaces. Macromolecules 13, 34–41 (1980).
Massou, S., Albigot, R. & Prats, M. Carboxyfluorescein fluorescence experiments. Biochem. Educ. 28, 171–173 (2000).
Van der Wel, C. et al. Preparation of colloidal organosilica spheres through spontaneous emulsification. Langmuir 33, 8174–8180 (2017).
Li, B. et al. Metal-organic framework based upon the synergy of a Bronsted acid framework and Lewis acid centers as a highly efficient heterogeneous catalyst for fixed-bed reactions. J. Am. Chem. Soc. 137, 4243–4248 (2015).
Wei, W. et al. Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier. J. Am. Chem. Soc. 130, 15808–15810 (2008).
Acknowledgements
This work was supported in part by the Innovation and Technology Commission (HKSAR, China) to the State Key Laboratory of Synthetic Chemistry, and the Hong Kong Research Grants Council General Research Fund (grant nos. GRF17305917, GRF17303015 and GRF17304618), the Seed Funding for Interdisciplinary Research (University of Hong Kong), the URC Strategic Research Theme on New Materials (University of Hong Kong), the Science Technology and Innovation Programme of Shenzhen (JCYJ20170818141618963), the Shenzhen-Hong Kong Innovation Circle Programme (SGDX2019081623341332) and the National Natural Science Foundation of China (no. 11874397).
Author information
Authors and Affiliations
Contributions
C.W. and J.T. conceived and designed the project. C.W., J.D., L.G. and J.L. prepared the samples. C.W., J.D. and J.T. conducted most of the measurements and analysis. X.L. helped with COMSOL simulation. M.Y. performed the coarse-grained simulation and analysis. C.W. and J.T. wrote most of the manuscript. J.D. and M.Y. participated in the manuscript revision. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14, Discussion and Table 1.
Supplementary Video 1
A. Self-propulsion of the ZnO nanorod in water. B. Assembly of sulfonated PS in water. C. Chemotaxis of sulfonated PS towards the Zn2+ source.
Supplementary Video 2
A. Synergistic interaction between ZnO nanorods and sulfonated PS. B. Attraction, rotation and expulsion of sulfonated PS.
Supplementary Video 3
A. Motion of sulfonated PS with an immobilized ZnO nanorod. B. The sulfonated PS depletion region formation around a ZnO nanorod. C. Interaction between a CaCO3 microcube and sulfonated PS. D. Interaction between a MIL-101 (Fe) nanoparticle and ZnO nanorod. E. Interaction between Janus Pt-TPM and a PS microsphere.
Supplementary Video 4
A. 3D pH profile near a ZnO nanorod during the interaction between a ZnO nanorod and sulfonated PS B. 3D trajectory of sulfonated PS when interacting with a ZnO nanorod fixed on the substrate.
Supplementary Video 5
A. Complex formation with an active ZnO nanorod and sulfonated PS. B. Cluster formation of ZnO nanorod–sulfonated PS. C. Attraction and alignment behaviour of free sulfonated PS. D. Large-scale swarming behaviour of the ZnO nanorod–sulfonated PS mixture.
Supplementary Video 6
A. Simulation of a ZnO–sulfonated PS system with chemical communication and electroosmosis interaction. B. Simulation of a ZnO–sulfonated PS system without chemical communication. C. Simulation of a ZnO–sulfonated PS system without electroosmosis interaction. D. Simulation of a ZnO–sulfonated PS system in circular confinement.
Supplementary Video 7
A. Phase segregation of active ZnO–sulfonated PS in a glass petri dish. B. Diffusion of passive PS microspheres in a glass petri dish. C. Active Pt-TPM Janus microspheres in a glass petri dish. D. Active ZnO nanorods in a glass petri dish. E. Phase segregation of active ZnO–sulfonated PS in a glass petri dish with 10 μM EDTA. F. Phase segregation of active ZnO–sulfonated PS in a glass petri dish with a sulfonated PS-coated quartz plate and a blank quartz plate for comparison. G. Phase segregation of active ZnO–sulfonated PS in a glass petri dish with fixed ZnO nanorods loaded agarose disc plate and a blank agarose disc for comparison.
Supplementary Video 8
Quorum decision-making of ZnO–sulfonated PS in four cookie moulds.
Source data
41565_2020_825_MOESM10_ESM.xlsx
Source Data Fig. 2 Figure 2c, The migration speed of the ZnO nanorod shown, where the shaded speed spikes correspond to the transient ZnO assembly with sulfonated PS. Figure 2e, Relationship of the speed of sulfonated PS with their separation distance from the ZnO nanorod. The error bars represent the standard deviation of the speed from multiple particles (n = 50).
Source Data Fig. 3
The power-law dependence between ion flux density and interparticle distance, where the error bars are the standard deviation of multiple sulfonated PS (n = 20).
Rights and permissions
About this article
Cite this article
Wu, C., Dai, J., Li, X. et al. Ion-exchange enabled synthetic swarm. Nat. Nanotechnol. 16, 288–295 (2021). https://doi.org/10.1038/s41565-020-00825-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-00825-9
This article is cited by
-
Photochromism from wavelength-selective colloidal phase segregation
Nature (2023)
-
Autonomous environment-adaptive microrobot swarm navigation enabled by deep learning-based real-time distribution planning
Nature Machine Intelligence (2022)
-
Visible light-regulated BiVO4-based micromotor with biomimetic ‘predator-bait’ behavior
Journal of Materials Science (2022)
-
Light hybrid micro/nano-robots: From propulsion to functional signals
Nano Research (2022)