Abstract
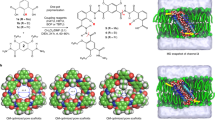
Inspired by biological proteins, artificial water channels (AWCs) can be used to overcome the performances of traditional desalination membranes. Their rational incorporation in composite polyamide provides an example of biomimetic membranes applied under representative reverse osmosis desalination conditions with an intrinsically high water-to-salt permeability ratio. The hybrid polyamide presents larger voids and seamlessly incorporates I–quartet AWCs for highly selective transport of water. These biomimetic membranes can be easily scaled for industrial standards (>m2), provide 99.5% rejection of NaCl or 91.4% rejection of boron, with a water flux of 75 l m−2 h−1 at 65 bar and 35,000 ppm NaCl feed solution, representative of seawater desalination. This flux is more than 75% higher than that observed with current state-of-the-art membranes with equivalent solute rejection, translating into an equivalent reduction of the membrane area for the same water output and a roughly 12% reduction of the required energy for desalination.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Mekonnen, M. M. & Hoekstra, A. Y. Four billion people facing severe water scarcity. Sci. Adv. 2, e1500323 (2016).
Eliasson, J. The rising pressure of global water shortages. Nature 517, 6–7 (2015).
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Cadotte, J. E., Petersen, R. J., Larson, R. E. & Erickson, E. E. A new thin-film composite seawater reverse osmosis membrane. Desalination 32, 25–31 (1980).
Karan, S., Jiang, Z. & Livingston, A. G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 348, 1347–1351 (2015).
Chowdhury, M. R., Steffes, J., Huey, B. D. & McCutcheon, J. R. 3D printed polyamide membranes for desalination. Science 361, 682–686 (2018).
Tan, Z., Chen, S., Peng, X., Zhang, L. & Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 360, 518–521 (2018).
Jeong, B. H. et al. Interfacial polymerization of thin film nanocomposites: a new concept for reverse osmosis membranes. J. Memb. Sci. 294, 1–7 (2007).
Duan, J. et al. High-performance polyamide thin-film-nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8. J. Memb. Sci. 476, 303–310 (2015).
Ratto, T. V., Holt, J. K. & Szmodis, A. W. Asymmetric nanotube containing membranes. US patent 7,993,524 (2011).
Werber, J. R., Deshmukh, A. & Elimelech, M. The critical need for increased selectivity, not increased water permeability, for desalination membranes. Environ. Sci. Techol. Lett. 3, 112–120 (2016).
Agre, P. Aquaporin water channels (Nobel lecture). Angew. Chem. Int. Ed. 43, 4278–4290 (2004).
Eriksson, U. K. et al. Subangstrom resolution X-ray structure details aquaporin–water interactions. Science 340, 1346–1349 (2013).
Tang, C. Y., Zhao, Y., Wang, R., Hélix-Nielsen, C. & Fane, A. G. Desalination by biomimetic aquaporin membranes: review of status and prospects. Desalination 308, 34–40 (2013).
Barboiu, M. Artificial water channels. Angew. Chem. Int. Ed. 51, 11674–11676 (2012).
Barboiu, M. & Gilles, A. From natural to bioassisted and biomimetic artificial water channel systems. Acc. Chem. Res. 46, 2814–2823 (2013).
Leduc, Y. et al. Imidazole-quartet water and proton dipolar channels. Angew. Chem. Int. Ed. 50, 11366–11372 (2011).
Hu, X. B., Chen, Z., Tang, G., Hou, J. L. & Li, Z. T. Single-molecular artificial transmembrane water channels. J. Am. Chem. Soc. 134, 8384–8387 (2012).
Si, W. et al. Selective artificial transmembrane channels for protons by formation of water wires. Angew. Chem. Int. Ed. 50, 12564–12568 (2011).
Licsandru, E. et al. Salt-excluding artificial water channels exhibiting enhanced dipolar water and proton translocation. J. Am. Chem. Soc. 138, 5403–5409 (2016).
Tunuguntla, R. H. et al. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 357, 792–796 (2017).
Kocsis, I. et al. Oriented chiral water wires in artificial transmembrane channels. Sci. Adv. 4, eaao5603 (2018).
Werber, J. R. & Elimelech, M. Permselectivity limits of biomimetic desalination membranes. Sci. Adv. 4, eaar8266 (2018).
McGinnis, R. L. et al. Large-scale polymeric carbon nanotube membranes with sub-1.27-nm pores. Sci. Adv. 4, e1700938 (2018).
Mi, B. Graphene oxide membranes for ionic and molecular sieving. Science 343, 740–742 (2014).
Yang, Y. et al. Large-area graphene-nanomesh/carbon-nanotube hybrid membranes for ionic and molecular nanofiltration. Science 364, 1057–1062 (2019).
Shen, Y. X. et al. Achieving high permeability and enhanced selectivity for Angstrom-scale separations using artificial water channel membranes. Nat. Commun. 9, 2294 (2018).
Lin, L., Lopez, R., Ramon, G. Z. & Coronell, O. Investigating the void structure of the polyamide active layers of thin-film composite membranes. J. Memb. Sci. 497, 365–376 (2016).
Pacheco, F., Sougrat, R., Reinhard, M., Leckie, J. O. & Pinnau, I. 3D visualization of the internal nanostructure of polyamide thin films in RO membranes. J. Memb. Sci. 501, 33–44 (2016).
Wong, M. C. Y., Lin, L., Coronell, O., Hoek, E. M. V. & Ramon, G. Z. Impact of liquid-filled voids within the active layer on transport through thin-film composite membranes. J. Memb. Sci. 500, 124–135 (2016).
Li, Y. et al. Probing flow activity in polyamide layer of reverse osmosis membrane with nanoparticle tracers. J. Memb. Sci. 534, 9–17 (2017).
Acknowledgements
This work was supported by Agence Nationale de la Recherche grant nos. ANR-18-CE06-0004-02, WATERCHANNELS and ERANETMED2-72-357 IDEA. The authors thank D. Cot (University of Montpellier) for SEM experiments, F. Ricceri (Politecnico di Torino) for help with filtration experiments and M. Deleanu (University of Montpellier) for help with the organic synthesis of HC6 and characterization.
Author information
Authors and Affiliations
Contributions
M.B. conceived the project and designed the experiments. M.D.V. fabricated the membranes, performed IR, EDX characterization and SEM analysis. L.-B.H. performed X-ray powder diffraction analysis. M.D.V. and A.T. designed and performed the filtration experiments. S.P.N. designed and V.-E.M., S.C. and R.S., performed DLS, SAXS and TEM experiments and conducted the experimental analysis. M.B. wrote the manuscript with input from all authors. All authors discussed the results and commented on the manuscript
Corresponding author
Ethics declarations
Competing interests
M.B., M.D.V. and A.T. are inventors on a provisional patent application submitted in 2019 by the SATT AxLR on behalf of the Centre National de la Recherche Scientifique (CNRS) for the design, synthesis and performances of the membrane materials in this study.
Additional information
Peer review information Nature Nanotechnology thanks Isabel Escobar, Jun-li Hou and Claus Helix Nielsen for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Figs 1–12, Tables 1–8, Methods, discussion and refs. S1 and S2.
Supplementary Video 1
The sum of tilted images of the sample TFC with different angles of TEM tomography.
Supplementary Video 2
The sum of tilted images of the sample TFC–HC6 with different angles of TEM tomography.
Rights and permissions
About this article
Cite this article
Di Vincenzo, M., Tiraferri, A., Musteata, VE. et al. Biomimetic artificial water channel membranes for enhanced desalination. Nat. Nanotechnol. 16, 190–196 (2021). https://doi.org/10.1038/s41565-020-00796-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-00796-x
This article is cited by
-
Precision ion separation via self-assembled channels
Nature Communications (2024)
-
Self-assembled dendrimer polyamide nanofilms with enhanced effective pore area for ion separation
Nature Communications (2024)
-
Electrolyte-assisted polarization leading to enhanced charge separation and solar-to-hydrogen conversion efficiency of seawater splitting
Nature Catalysis (2024)
-
Thin-film composite membrane for desalination containing a sulfonated UiO-66 material
Journal of Materials Science (2023)
-
Multifunctional graphene heterogeneous nanochannel with voltage-tunable ion selectivity
Nature Communications (2022)