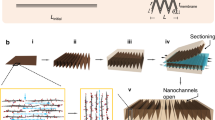
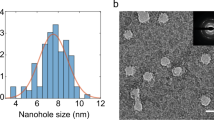
Abstract
Membranes are ubiquitous in nature with primary functions that include adaptive filtering and selective transport of chemical/molecular species. Being critical to cellular functions, they are also fundamental in many areas of science and technology. Of particular importance are the adaptive and programmable membranes that can change their permeability or selectivity depending on the environment. Here, we explore implementation of such biological functions in artificial membranes and demonstrate two-dimensional self-assembled heterostructures of graphene oxide and polyamine macromolecules, forming a network of ionic channels that exhibit regulated permeability of water and monovalent ions. This permeability can be tuned by a change of pH or the presence of certain ions. Unlike traditional membranes, the regulation mechanism reported here relies on specific interactions between the membranes’ internal components and ions. This allows fabrication of membranes with programmable, predetermined permeability and selectivity, governed by the choice of components, their conformation and their charging state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author on reasonable request.
References
Noireaux, V. & Libchaber, A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl Acad. Sci. USA 101, 17669–17674 (2004).
Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015).
Rothfield, L. I. Structure and Function of Biological Membranes (Academic Press, 1971).
Sowerby, S. J., Stockwell, P. A., Heckl, W. M. & Petersen, G. B. Self-programmable, self-assembling two-dimensional genetic matter. Orig. Life Evol. Biosph. 30, 81–99 (2000).
Nitta, N., Wu, F. X., Lee, J. T. & Yushin, G. Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015).
Wang, J. L. & Zhuang, S. T. Removal of cesium ions from aqueous solutions using various separation technologies. Rev. Environ. Sci. Biotechnol. 18, 231–269 (2019).
Gopinadhan, K. et al. Complete steric exclusion of ions and proton transport through confined monolayer water. Science 363, 145–148 (2019).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Su, Y. et al. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nat. Commun. 5, 4843 (2014).
Chen, L. et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550, 415–418 (2017).
Nair, R. R., Wu, H. A., Jayaram, P. N., Grigorieva, I. V. & Geim, A. K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 335, 442–444 (2012).
Hong, S. et al. Scalable graphene-based membranes for ionic sieving with ultrahigh charge selectivity. Nano Lett. 17, 728–732 (2017).
Dikin, D. A. et al. Preparation and characterization of graphene oxide paper. Nature 448, 457–460 (2007).
Kotov, N. A., Dekany, I. & Fendler, J. H. Ultrathin graphite oxide–polyelectrolyte composites prepared by self-assembly: transition between conductive and non-conductive states. Adv. Mater. 8, 637–641 (1996).
Kulkarni, D. D., Choi, I., Singamaneni, S. & Tsukruk, V. V. Graphene oxide–polyelectrolyte nanomembranes. ACS Nano 4, 4667–4676 (2010).
Wei, C. F. & Lintilhac, P. M. Loss of stability: a new look at the physics of cell wall behavior during plant cell growth. Plant Physiol. 145, 763–772 (2007).
Burgert, I. & Fratzl, P. Mechanics of the Expanding Cell Wall 191–215 (Springer-Verlag, 2006).
Peters, W. S., Hagemann, W. & Tomos, A. D. What makes plants different? Principles of extracellular matrix function in ‘soft’ plant tissues. Comp. Biochem. Physiol. A 125, 151–167 (2000).
Sun, P. Z., Wang, K. L. & Zhu, H. W. Recent developments in graphene-based membranes: structure, mass-transport mechanism and potential applications. Adv. Mater. 28, 2287–2310 (2016).
Liu, G. P., Jin, W. Q. & Xu, N. P. Graphene-based membranes. Chem. Soc. Rev. 44, 5016–5030 (2015).
Qiu, L. et al. Controllable corrugation of chemically converted graphene sheets in water and potential application for nanofiltration. Chem. Commun. 47, 5810–5812 (2011).
Andreeva, D. V., Fix, D., Mohwald, H. & Shchukin, D. G. Self-healing anticorrosion coatings based on pH-sensitive polyelectrolyte/inhibitor sandwichlike nanostructures. Adv. Mater. 20, 2789–2794 (2008).
Shepherd, E. J. & Kitchener, J. A. The ionization of ethyleneimine and polyethyleneimine. J. Chem. Soc., 2448–2452 (1956).
Konkena, B. & Vasudevan, S. Understanding aqueous dispersibility of graphene oxide and reduced graphene oxide through pKa measurements. J. Phys. Chem. Lett. 3, 867–872 (2012).
Farhat, T., Yassin, G., Dubas, S. T. & Schlenoff, J. B. Water and ion pairing in polyelectrolyte multilayers. Langmuir 15, 6621–6623 (1999).
Cath, T. Y., Childress, A. E. & Elimelech, M. Forward osmosis: principles, applications, and recent developments. J. Membr. Sci. 281, 70–87 (2006).
Petrucci, R. H., Herring, F. G., Madura, J. D. & Bissonnette, C. General Chemistry: Principles and Modern Applications 577–580 (Pearson Education, 2016).
Yeh, C. N., Raidongia, K., Shao, J. J., Yang, Q. H. & Huang, J. X. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 7, 166–170 (2015).
Itano, K., Choi, J. Y. & Rubner, M. F. Mechanism of the pH-induced discontinuous swelling/deswelling transitions of poly(allylamine hydrochloride)-containing polyelectrolyte multilayer films. Macromolecules 38, 3450–3460 (2005).
Lutzenkirchen, J. et al. Potentiometric titrations as a tool for surface charge determination. Croat. Chem. Acta 85, 391–417 (2012).
Katchalsky, A. & Spitnik, P. Potentiometric titrations of polymethacrylic acid. J. Polym. Sci. 2, 432–446 (1947).
Delgado, A. V., Gonzalez-Caballero, F., Hunter, R. J., Koopal, L. K. & Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 309, 194–224 (2007).
Israelachvili, J. N. Intermolecular and Surface Forces (Elsevier, 2011).
Atkins, P. W. & de Paula, J. Atkins’ Physical Chemistry 8th edn (Oxford Univ. Press, 2006).
Krasemann, L. & Tieke, B. Selective ion transport across self-assembled alternating multilayers of cationic and anionic polyelectrolytes. Langmuir 16, 287–290 (2000).
Harris, J. J., DeRose, P. M. & Bruening, M. L. Synthesis of passivating, nylon-like coatings through cross-linking of ultrathin polyelectrolyte films. J. Am. Chem. Soc. 121, 1978–1979 (1999).
Cohen-Tanugi, D. & Grossman, J. C. Water desalination across nanoporous graphene. Nano Lett. 12, 3602–3608 (2012).
Han, Y., Xu, Z. & Gao, C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 23, 3693–3700 (2013).
Hu, M. & Mi, B. X. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 47, 3715–3723 (2013).
Lerf, A. et al. Hydration behavior and dynamics of water molecules in graphite oxide. J. Phys. Chem. Solids 67, 1106–1110 (2006).
Boukhvalov, D. W., Katsnelson, M. I. & Son, Y. W. Origin of anomalous water permeation through graphene oxide membrane. Nano Lett. 13, 3930–3935 (2013).
Koenig, S. P., Wang, L. D., Pellegrino, J. & Bunch, J. S. Selective molecular sieving through porous graphene. Nat. Nanotechnol. 7, 728–732 (2012).
Furutani, Y., Shimizu, H., Asai, Y., Oiki, S. & Kandori, H. Specific interactions between alkali metal cations and the KcsA channel studied using ATR-FTIR spectroscopy. Biophys. Physicobiol. 12, 37–45 (2015).
Rose, L. & Jenkins, A. T. A. The effect of the ionophore valinomycin on biomimetic solid supported lipid DPPTE/EPC membranes. Bioelectrochemistry 70, 387–393 (2007).
Yeagle, P. L. The Membranes of Cells (Elsevier Science, 2016).
Acknowledgements
We thank NRF (Singapore) for financial support through the project Medium-Sized Centre programme R-723-000-001-281, and RSF (Russian Federation) for grant no. 19-19-00508. M.T. thanks the Director’s Senior Research Fellowship of the Centre. K.S.N. also acknowledges support from EU Flagship Programs (Graphene CNECTICT-604391 and 2D-SIPC Quantum Technology), European Research Council Synergy Grant Hetero2D, the Royal Society and EPSRC grants EP/N010345/1, EP/P026850/1 and EP/S030719/1.
Author information
Authors and Affiliations
Contributions
D.V.A., M.T. and K.S.N. conceived and designed the experiments. A.N., M.C.F.C., P.V.C., M.H., K.Y., S.C., S.W.C. and U.M. performed the experiments. A.H.C.N. contributed the materials and analysis tools. D.V.A., M.T. and K.S.N. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Haiping Fang, Ho Bum Park and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–17, Discussion, Methods, Theory and Table 1.
Rights and permissions
About this article
Cite this article
Andreeva, D.V., Trushin, M., Nikitina, A. et al. Two-dimensional adaptive membranes with programmable water and ionic channels. Nat. Nanotechnol. 16, 174–180 (2021). https://doi.org/10.1038/s41565-020-00795-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-00795-y
This article is cited by
-
Two-dimensional non-linear hydrodynamics and nanofluidics
Communications Physics (2023)
-
Scalable high yield exfoliation for monolayer nanosheets
Nature Communications (2023)
-
High-surface-area functionalized nanolaminated membranes for energy-efficient nanofiltration and desalination in forward osmosis
Nature Water (2023)
-
Graphene oxide classification and standardization
Scientific Reports (2023)
-
Graphene oxide patchwork membranes
Nature Nanotechnology (2021)