Abstract
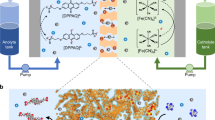
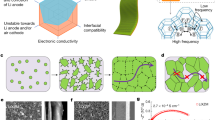
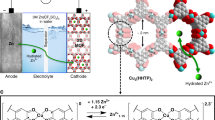
Rechargeable organic batteries show great potential as a low-cost, sustainable and mass-producible alternatives to current transition-metal-based cells; however, serious electrode dissolution issues and solubilization of organic redox intermediates (shuttle effect) have plagued the capacity retention and cyclability of these cells. Here we report on the use of a metal–organic framework (MOF) gel membrane as a separator for organic batteries. The homogeneous micropores, intrinsic of the MOF-gel separator, act as permselective channels for targeted organic intermediates, thereby mitigating the shuttling problem without sacrificing power. A battery using a MOF-gel separator and 5,5′-dimethyl-2,2′-bis-p-benzoquinone (Me2BBQ) as the electrode displays high cycle stability with capacity retention of 82.9% after 2,000 cycles, corresponding to a capacity decay of ~0.008% per cycle, with a discharge capacity of ~171 mA h g−1 at a current density of 300 mA g−1. The molecular and ionic sieving capabilities of MOF-gel separators promise general applicability, as pore size can be tuned to specific organic electrode materials. The use of MOF-gel separators to prevent side reactions of soluble organic redox intermediates could lead to the development of rechargeable organic batteries with high energy density and long cycling life.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data in the article are available from the corresponding author on reasonable request.
References
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J. M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Larcher, D. & Tarascon, J. M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2015).
Peng, C. X. et al. Reversible multi-electron redox chemistry of π-conjugated N-containing heteroaromatic molecule-based organic cathodes. Nat. Energy 2, 17074 (2017).
Poizot, P. & Dolhem, F. Clean energy new deal for a sustainable world: from non-CO2 generating energy sources to greener electrochemical storage devices. Energy Environ. Sci. 4, 2003–2019 (2011).
Song, Z. P. & Zhou, H. S. Towards sustainable and versatile energy storage devices: an overview of organic electrode materials. Energy Environ. Sci. 6, 2280–2301 (2013).
Liang, Y. L., Zhang, P., Yang, S. Q., Tao, Z. L. & Chen, J. Fused heteroaromatic organic compounds for high-power electrodes of rechargeable lithium batteries. Adv. Energy Mater. 3, 600–605 (2013).
Bhosale, M. E., Chae, S., Kim, J. M. & Choi, J. Y. Organic small molecules and polymers as an electrode material for rechargeable lithium ion batteries. J. Mater. Chem. A 6, 19885–19911 (2018).
Nokami, T. et al. Polymer-bound pyrene-4,5,9,10-tetraone for fast-charge and -discharge lithium-ion batteries with high capacity. J. Am. Chem. Soc. 134, 19694–19700 (2012).
Song, Z. P. et al. Polyanthraquinone as a reliable organic electrode for stable and fast lithium storage. Angew. Chem. Int. Ed. Engl. 54, 13947–13951 (2015).
Muench, S. et al. Polymer-based organic batteries. Chem. Rev. 116, 9438–9484 (2016).
Wang, S. et al. Exfoliation of covalent organic frameworks into few-layer redox-active nanosheets as cathode materials for lithium-ion batteries. J. Am. Chem. Soc. 139, 4258–4261 (2017).
Luo, Z. Q. et al. A microporous covalent-organic framework with abundant accessible carbonyl groups for lithium-ion batteries. Angew. Chem. Int. Ed. Engl. 57, 9443–9446 (2018).
Sieuw, L. et al. A H-bond stabilized quinone electrode material for Li-organic batteries: the strength of weak bonds. Chem. Sci. 10, 418–426 (2019).
Jouhara, A. et al. Raising the redox potential in carboxyphenolate-based positive organic materials via cation substitution. Nat. Commun. 9, 4401 (2018).
Kim, H. et al. High energy organic cathode for sodium rechargeable batteries. Chem. Mater. 27, 7258–7264 (2015).
Zhang, K., Guo, C., Zhao, Q., Niu, Z. & Chen, J. High-performance organic lithium batteries with an ether-based electrolyte and 9,10-anthraquinone (AQ)/CMK-3 cathode. Adv. Sci. 2, 1500018 (2015).
Huang, W. et al. Quasi-solid-state rechargeable lithium-ion batteries with a Calix[4]quinone cathode and gel polymer electrolyte. Angew. Chem. Int. Ed. Engl. 52, 9162–9166 (2013).
Zhu, Z. et al. All-solid-state lithium organic battery with composite polymer electrolyte and pillar[5]quinone cathode. J. Am. Chem. Soc. 136, 16461–16464 (2014).
Lei, X. et al. Flexible lithium–air battery in ambient air with an in situ formed gel electrolyte. Angew. Chem. Int. Ed. Engl. 57, 16131–16135 (2018).
Chi, X. et al. Tailored organic electrode material compatible with sulfide electrolyte for stable all-solid-state sodium batteries. Angew. Chem. Int. Ed. Engl. 57, 2630–2634 (2018).
Lee, S. et al. Recent progress in organic electrodes for Li and Na rechargeable batteries. Adv. Mater. 30, 1704682 (2018).
Wang, S. et al. A robust large-pore zirconium carboxylate metal–organic framework for energy-efficient water-sorption-driven refrigeration. Nat. Energy 3, 985–993 (2018).
Katsoulidis, A. P. et al. Chemical control of structure and guest uptake by a conformationally mobile porous material. Nature 565, 213–217 (2019).
Furukawa, H. et al. Ultrahigh porosity in metal-organic frameworks. Science 329, 424–428 (2010).
Qiu, S. L., Xue, M. & Zhu, G. S. Metal-organic framework membranes: from synthesis to separation application. Chem. Soc. Rev. 43, 6116–6140 (2014).
Cao, L. et al. Channel-facilitated molecule and ion transport across polymer composite membranes. Chem. Soc. Rev. 46, 6725–6745 (2017).
Bachman, J. E., Smith, Z. P., Li, T., Xu, T. & Long, J. R. Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal–organic framework nanocrystals. Nat. Mater. 15, 845–849 (2016).
Bai, S. Y. et al. Distinct anion sensing by a 2D self-assembled Cu(I)-based metal–organic polymer with versatile visual colorimetric responses and efficient selective separations via anion exchange. J. Mater. Chem. A 1, 2970–2973 (2013).
Xia, B. Y. et al. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 1, 15006 (2016).
Chen, Y. et al. A solvent-free hot-pressing method for preparing metal–organic-framework coatings. Angew. Chem. Int. Ed. Engl. 55, 3419–3423 (2016).
Bai, S. Y. et al. High-power Li-metal anode enabled by metal-organic framework modified electrolyte. Joule 2, 2117–2132 (2018).
Yao, H. B. et al. Improved lithium–sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode–separator interface. Energy Environ. Sci. 7, 3381–3390 (2014).
Xu, J. J. et al. In situ construction of stable tissue-directed/reinforced bifunctional separator/protection film on lithium anode for lithium-oxygen batteries. Adv. Mater. 29, 1606552 (2017).
Bai, S. Y., Liu, X. Z., Zhu, K., Wu, S. C. & Zhou, H. S. Metal–organic framework-based separator for lithium–sulfur batteries. Nat. Energy 1, 16094 (2016).
Song, Z., Qian, Y., Otani, M. & Zhou, H. Stable Li–organic batteries with nafion-based sandwich-type separators. Adv. Energy Mater. 6, 1501780 (2016).
Denny, M. S. Jr, Moreton, J. C., Benz, L. & Cohen, S. M. Metal–organic frameworks for membrane-based separations. Nat. Rev. Mater. 1, 16078 (2016).
Koros, W. J. & Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 16, 289–297 (2017).
Park, H. B., Kamcev, J., Robeson, L. M., Elimelech, M. & Freeman, B. D. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017).
Li, W. et al. Ultrathin metal–organic framework membrane production by gel–vapour deposition. Nat. Commun. 8, 406 (2017).
Tian, T. et al. A sol-gel monolithic metal–organic framework with enhanced methane uptake. Nat. Mater. 17, 174–179 (2018).
Yokoji, T., Kameyama, Y., Maruyama, N. & Matsubara, H. High-capacity organic cathode active materials of 2,2′-bis-p-benzoquinone derivatives for rechargeable batteries. J. Mater. Chem. A 4, 5457–5466 (2016).
Miao, L. C. et al. The structure–electrochemical property relationship of quinone electrodes for lithium-ion batteries. Phys. Chem. Chem. Phys. 20, 13478–13484 (2018).
Fairen-Jimenez, D. et al. Opening the gate: framework flexibility in ZIF-8 explored by experiments and simulations. J. Am. Chem. Soc. 133, 8900–8902 (2011).
Wang, B., Cote, A. P., Furukawa, H., O’Keeffe, M. & Yaghi, O. M. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature 453, 207–211 (2008).
Huang, A. S., Liu, Q., Wang, N. Y., Zhu, Y. Q. & Caro, J. Bicontinuous zeolitic imidazolate framework ZIF-8@GO membrane with enhanced hydrogen selectivity. J. Am. Chem. Soc. 136, 14686–14689 (2014).
Schon, T. B., McAllister, B. T., Li, P. F. & Seferos, D. S. The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 45, 6345–6406 (2016).
Anh, N. V. & Williams, R. M. Bis-semiquinone (bi-radical) formation by photoinduced proton coupled electron transfer in covalently linked catechol–quinone systems: Aviram’s hemiquinones revisited. Photoch. Photobio. Sci. 11, 957–961 (2012).
Zhu, Y., Xiao, S., Shi, Y., Yang, Y. & Wu, Y. A trilayer poly(vinylidene fluoride)/polyborate/poly(vinylidene fluoride) gel polymer electrolyte with good performance for lithium ion batteries. J. Mater. Chem. A 1, 7790–7797 (2013).
Li, W. et al. A PEO-based gel polymer electrolyte for lithium ion batteries. RSC Adv. 7, 23494–23501 (2017).
Vadehra, G. S., Maloney, R. P., Garcia-Garibay, M. A. & Dunn, B. Naphthalene diimide based materials with adjustable redox potentials: evaluation for organic lithium-ion batteries. Chem. Mater. 26, 7151–7157 (2014).
Li, H. et al. 2,2′-Bis(3-hydroxy-1,4-naphthoquinone)/CMK-3 nanocomposite as cathode material for lithium-ion batteries. Inorg. Chem. Front. 1, 193–199 (2014).
Gaussian 16 Rev. B.01 (Gaussian Inc., 2016).
Lee, C. T., Yang, W. T. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Schafer, A., Huber, C. & Ahlrichs, R. Fully optimized contracted Gaussian-basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100, 5829–5835 (1994).
Acknowledgements
This work was financially supported by the Creative Materials Discovery Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2017M3D1A1039553). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (no. 2018R1A2A1A05079249), and project code (IBS-R006-A2). S.B. acknowledges the Korea Research Fellowship (KRF) Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (project no. 2018H1D3A1A01039450).
Author information
Authors and Affiliations
Contributions
K.K. and S.B. conceived the original idea for this work. S.B. designed and performed the experiments. B.K. performed the DFT calculation and discussed the main idea of the manuscript. C.K. and D.L. helped synthesize the electrode materials of Me2BBQ, BNQ and BHNQ. O.T. helped analyse the SEM images. H.P. helped analyse the ultraviolet–visible spectra. H.P. and J.K. helped the data analysis. S.B. wrote a draft of the manuscript, and K.K. revised it. All of the authors discussed the results of the manuscript. K.K. supervised all of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Birgit Esser, Kian Ping Loh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–43, Tables 1–4 and Notes 1 and 2.
Rights and permissions
About this article
Cite this article
Bai, S., Kim, B., Kim, C. et al. Permselective metal–organic framework gel membrane enables long-life cycling of rechargeable organic batteries. Nat. Nanotechnol. 16, 77–84 (2021). https://doi.org/10.1038/s41565-020-00788-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-00788-x
This article is cited by
-
Self-healable gels in electrochemical energy storage devices
Nano Research (2024)
-
Hydrothermal synthesis of polyimide-linked covalent organic frameworks towards ultrafast and stable cathodic sodium storage
Science China Chemistry (2024)
-
Recent advances in multifunctional metal-organic frameworks for lithium metal batteries
Science China Chemistry (2024)
-
Small-molecule organic electrode materials for rechargeable batteries
Science China Chemistry (2023)
-
Trimming the Degrees of Freedom via a K+ Flux Rectifier for Safe and Long-Life Potassium-Ion Batteries
Nano-Micro Letters (2023)