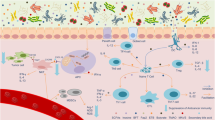
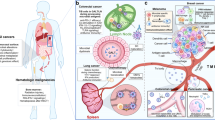
Abstract
The microbiome is emerging as a key player and driver of cancer. Traditional modalities to manipulate the microbiome (for example, antibiotics, probiotics and microbiota transplants) have been shown to improve efficacy of cancer therapies in some cases, but issues such as collateral damage to the commensal microbiota and consistency of these approaches motivates efforts towards developing new technologies specifically designed for the microbiome–cancer interface. Considering the success of nanotechnology in transforming cancer diagnostics and treatment, nanotechnologies capable of manipulating interactions that occur across microscopic and molecular length scales in the microbiome and the tumour microenvironment have the potential to provide innovative strategies for cancer treatment. As such, opportunities at the intersection of nanotechnology, the microbiome and cancer are massive. In this Review, we highlight key opportunistic areas for applying nanotechnologies towards manipulating the microbiome for the treatment of cancer, give an overview of seminal work and discuss future challenges and our perspective on this emerging area.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Turnbaugh, P. J. et al. The human microbiome project. Nature 449, 804 (2007).
Helmink, B. A., Khan, M. A. W., Hermann, A., Gopalakrishnan, V. & Wargo, J. A. The microbiome, cancer, and cancer therapy. Nat. Med. 25, 377–388 (2019). A recent review discussing the influence of gut microbiota on cancer therapy and current approaches targeting gut microbiome for cancer therapy.
Schwabe, R. F. & Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 13, 800 (2013). An article that summarizes the links between bacterial microbiota and cancer, many of the driving mechanisms, and strategies that involve targeting the microbiome for cancer prevention.
Hatakeyama, M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 4, 688 (2004).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013).
Arthur, J. C. et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123 (2012).
Bhatt, A. P., Redinbo, M. R. & Bultman, S. J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 67, 326–344 (2017).
Bullman, S. et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448 (2017). This paper demonstrated that Fusobacterium and its associated microbiome is maintained in distal metastases and motivates the need to target TAB.
Riquelme, E. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806e712 (2019).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013).
Derosa, L. et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 29, 1437–1444 (2018).
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Geller, L. T. et al. Potential role of intratumour bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
McQuade, J. L., Daniel, C. R., Helmink, B. A. & Wargo, J. A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 20, e7–e91 (2019).
Gopalakrishnan, V., Helmink, B. A., Spencer, C. N., Reuben, A. & Wargo, J. A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer cell 33, 570–580 (2018).
Young, V. B. Therapeutic manipulation of the microbiota: past, present, and considerations for the future. Clin. Microbiol. Infect. 22, 905–909 (2016).
Vargason, A. M. & Anselmo, A. C. Clinical translation of microbe-based therapies: Current clinical landscape and preclinical outlook. Bioeng. Transl. Med. 3, 124–137 (2018).
Mimee, M., Citorik, R. J. & Lu, T. K. Microbiome therapeutics—advances and challenges. Adv. Drug Deliv. Rev. 105, 44–54 (2016).
Jobin, C. Precision medicine using microbiota. Science 359, 32–34 (2018).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 1, 10–29 (2016).
Li, S.-D. & Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharmaceut. 5, 496–504 (2008).
Owens, D. E. III & Peppas, N. A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 307, 93–102 (2006).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Kamaly, N., Yameen, B., Wu, J. & Farokhzad, O. C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 116, 2602–2663 (2016).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 4, e10143 (2019).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnol. 2, 751–760 (2007).
Ma, L., Kohli, M. & Smith, A. Nanoparticles for combination drug therapy. ACS nano 7, 9518–9525 (2013).
Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013).
Moon, J. J., Huang, B. & Irvine, D. J. Engineering nano‐and microparticles to tune immunity. Adv. Mater. 24, 3724–3746 (2012).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature Biotechnol. 33, 941–951 (2015).
Hu, C. M. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015).
Rodriguez, P. L. et al. Minimal "self" peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 339, 971–975 (2013).
Petros, R. A. & DeSimone, J. M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 9, 615–627 (2010).
Albanese, A., Tang, P. S. & Chan, W. C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 14, 1–16 (2012).
Bertrand, N., Wu, J., Xu, X., Kamaly, N. & Farokhzad, O. C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 66, 2–25 (2014).
Tropini, C., Earle, K. A., Huang, K. C. & Sonnenburg, J. L. The gut microbiome: connecting spatial organization to function. Cell Host Microbe 21, 433–442 (2017).
Hua, S., Marks, E., Schneider, J. J. & Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine: NBM 11, 1117–1132 (2015).
Jin, K., Luo, Z., Zhang, B. & Pang, Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm. Sin. B 8, 23–33 (2018).
Lai, S. K., Wang, Y.-Y. & Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 61, 158–171 (2009).
Angsantikul, P. et al. Coating nanoparticles with gastric epithelial cell membrane for targeted antibiotic delivery against Helicobacter pylori infection. Adv. Ther. (Weinh) 1, 1800016 (2018). This manuscript describes a strategy that facilitates antibiotic delivery to specific bacteria via a targeted nanotechnology.
Pridgen, E. M. et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci. Transl. Med. 5, 213ra167 (2013).
Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 4, 437–447 (2004).
Poon, Z., Chang, D., Zhao, X. & Hammond, P. T. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumour hypoxia. ACS nano 5, 4284–4292 (2011).
Song, W. et al. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat. Commun. 9, 2237 (2018).
Hu, S. et al. The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PloS one 6, e16221 (2011).
Postler, T. S. & Ghosh, S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 26, 110–130 (2017). A comprehensive review that discusses how microbial metabolites affect the host immune system, thereby highlighting a multitude of targets for manipulation of microbiome signals.
Song, W. T. et al. Trapping of Lipopolysaccharide to Promote Immunotherapy against Colorectal Cancer and Attenuate Liver Metastasis. Adv. Mater. 30, 1805007 (2018). In this study, a nanotechnology was used to block signals from the gut microbiome to facilitate and enhance cancer immunotherapy.
Dapito, D. H. et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer cell 21, 504–516 (2012).
Goodwin, T. J., Zhou, Y., Musetti, S. N., Liu, R. & Huang, L. Local and transient gene expression primes the liver to resist cancer metastasis. Sci. Transl. Med. 8, 364ra153 (2016).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013).
Pushalkar, S. et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 8, 403–416 (2018).
Yu, T. et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563 (2017).
Xiong, M.-H. et al. Differential anticancer drug delivery with a nanogel sensitive to bacteria-accumulated tumor artificial environment. ACS Nano 7, 10636–10645 (2013). This study provides an approach for using bacterial metabolism in TAB as a signal for triggering drug release from nanoparticles for selectively killing cancer cells.
Singh, N. et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 (2014).
Wang, T. et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 6, 320 (2012).
Antimicrobial Resistance: Global Report on Surveillance (World Health Organization, 2014).
Vangay, P., Ward, T., Gerber, J. S. & Knights, D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 17, 553–564 (2015).
Francescone, R., Hou, V. & Grivennikov, S. I. Microbiome, inflammation and cancer. Cancer J. 20, 181–189 (2014).
Wu, N. et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb. Ecol. 66, 462–470 (2013).
Ramteke, S., Ganesh, N., Bhattacharya, S. & Jain, N. K. Amoxicillin, clarithromycin, and omeprazole based targeted nanoparticles for the treatment of H. pylori. J. Drug Target 17, 225–234 (2009).
Gao, W., Thamphiwatana, S., Angsantikul, P. & Zhang, L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 6, 532–547 (2014).
Vargas-Reus, M. A., Memarzadeh, K., Huang, J., Ren, G. G. & Allaker, R. P. Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int. J. Antimicrob. Agents 40, 135–139 (2012).
Lu, Z., Rong, K., Li, J., Yang, H. & Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 24, 1465–1471 (2013).
Hajipour, M. J. et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 30, 499–511 (2012).
Roberfroid, M. et al. Prebiotic effects: metabolic and health benefits. Brit. J. Nutr. 104, S1–S63 (2010).
Kutter, E. et al. Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11, 69–86 (2010).
Zheng, D.-W. et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 3, 717–728 (2019). This study describes a phage-primed approach for targeting nanoparticles to microbiota burdened tumours and outlines a possible workflow in developing personalized nanotechnologies against TAB.
Wu, W., Yang, Y. & Sun, G. Recent insights into antibiotic resistance in Helicobacter pylori eradication. Gastroenterol. Res. Pract. https://doi.org/10.1155/2012/723183 (2012).
Nyfors, S., Könönen, E., Syrjänen, R., Komulainen, E. & Jousimies-Somer, H. Emergence of penicillin resistance among Fusobacterium nucleatum populations of commensal oral flora during early childhood. J. Antimicrob. Chemother. 51, 107–112 (2003).
Tenover, F. C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 119, S3–S10 (2006).
Li, P., Li, J., Wu, C., Wu, Q. & Li, J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 16, 1912 (2005).
Alon, U., Surette, M. G., Barkai, N. & Leibler, S. Robustness in bacterial chemotaxis. Nature 397, 168 (1999).
Danino, T. et al. Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 7, 289ra284 (2015).
Forbes, N. S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 10, 785 (2010).
Akin, D. et al. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nature Nanotechnol. 2, 441 (2007).
Hu, Q. et al. Engineering Nanoparticle-Coated Bacteria as Oral DNA Vaccines for Cancer Immunotherapy. Nano Lett. 15, 2732–2739 (2015).
Fan, J.-X. et al. Bacteria-mediated tumor therapy utilizing photothermally-controlled TNF-α expression via oral administration. Nano Lett. 18, 2373–2380 (2018).
Hosseinidoust, Z. et al. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv. Drug Deliv. Rev. 106, 27–44 (2016).
Song, Q. et al. A probiotic spore-based oral autonomous nanoparticles generator for cancer therapy. Adv. Mater. 31, 1903793 (2019). This manuscript describes an approach utilizing natural functions of spore forming bacteria to autonomously produce nanoparticles in the intestine.
Schuerle, S. et al. Synthetic and living micropropellers for convection-enhanced nanoparticle transport. Sci. Adv. 5, eaav4803 (2019). This study develops a bacteria-inspired approach for improving nanoparticle delivery across tumour barriers using a microfabricated technology that mimics the collective behaviour of microbe communities.
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Qiu, K., Durham, P. G. & Anselmo, A. C. Inorganic nanoparticles and the microbiome. Nano Res. 11, 4936–4954 (2018).
Pietroiusti, A., Magrini, A. & Campagnolo, L. New frontiers in nanotoxicology: gut microbiota/microbiome-mediated effects of engineered nanomaterials. Toxicol. Appl. Pharmacol. 299, 90–95 (2016).
McClements, D. J. & Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food 1, 6 (2017).
Bouwmeester, H., van der Zande, M. & Jepson, M. A. Effects of food‐borne nanomaterials on gastrointestinal tissues and microbiota. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 10, e1481 (2018).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012).
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580 (2008).
Sanvicens, N., Pastells, C., Pascual, N. & Marco, M.-P. Nanoparticle-based biosensors for detection of pathogenic bacteria. Trends Analyt. Chem. 28, 1243–1252 (2009).
De Luca, F. & Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 195, 74–85 (2019).
Li, B., Selmi, C., Tang, R., Gershwin, M. E. & Ma, X. The microbiome and autoimmunity: a paradigm from the gut–liver axis. Cell. Mol. Immunol. 15, 595–609 (2018).
Rogers, G. B. et al. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol. Psychiatry 21, 738–748 (2016).
Griffiths, J. A. & Mazmanian, S. K. Emerging evidence linking the gut microbiome to neurologic disorders. Genome Med. 10, 98 (2018).
Hansen, J. J. & Sartor, R. B. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr. Treat. Options. Gastroenterol. 13, 105–120 (2015).
Lee, Y. et al. Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. https://doi.org/10.1038/s41563-019-0462-9 (2019).
Acknowledgements
W.S. would like to acknowledge support from the National Natural Science Foundation of China (51673185, 51973215). A.C.A. would like to acknowledge support from the Carolina Center of Cancer Nanotechnology Excellence (C-CCNE) Pilot Grant Program supported by the National Institutes of Health (NIH) National Cancer Institute (5U54CA198999-04). L.H. is supported by NIH grant CA198999.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, W., Anselmo, A.C. & Huang, L. Nanotechnology intervention of the microbiome for cancer therapy. Nat. Nanotechnol. 14, 1093–1103 (2019). https://doi.org/10.1038/s41565-019-0589-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0589-5
This article is cited by
-
Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation
Molecular Cancer (2023)
-
Self-polymerized platinum (II)-Polydopamine nanomedicines for photo-chemotherapy of bladder Cancer favoring antitumor immune responses
Journal of Nanobiotechnology (2023)
-
Modulating gut microbiota using nanotechnology to increase anticancer efficacy of the treatments
Macromolecular Research (2023)
-
Nanomicrobiology: Emerging Trends in Microbial Synthesis of Nanomaterials and Their Applications
Journal of Cluster Science (2023)
-
Exercise and the gut microbiome: implications for supportive care in cancer
Supportive Care in Cancer (2023)