Abstract
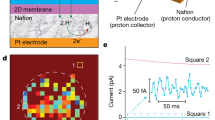
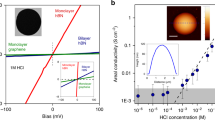
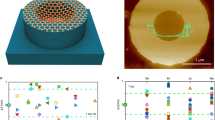
Monolayers of graphene and hexagonal boron nitride (hBN) are highly permeable to thermal protons1,2. For thicker two-dimensional (2D) materials, proton conductivity diminishes exponentially, so that, for example, monolayer MoS2 that is just three atoms thick is completely impermeable to protons1. This seemed to suggest that only one-atom-thick crystals could be used as proton-conducting membranes. Here, we show that few-layer micas that are rather thick on the atomic scale become excellent proton conductors if native cations are ion-exchanged for protons. Their areal conductivity exceeds that of graphene and hBN by one to two orders of magnitude. Importantly, ion-exchanged 2D micas exhibit this high conductivity inside the infamous gap for proton-conducting materials3, which extends from ∼100 °C to 500 °C. Areal conductivity of proton-exchanged monolayer micas can reach above 100 S cm−2 at 500 °C, well above the current requirements for the industry roadmap4. We attribute the fast proton permeation to ~5-Å-wide tubular channels that perforate micas’ crystal structure, which, after ion exchange, contain only hydroxyl groups inside. Our work indicates that there could be other 2D crystals5 with similar nanometre-scale channels, which could help close the materials gap in proton-conducting applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors on reasonable request.
References
Hu, S. et al. Proton transport through one-atom-thick crystals. Nature 516, 227–230 (2014).
Lozada-Hidalgo, M. et al. Sieving hydrogen isotopes through two-dimensional crystals. Science 351, 68–70 (2016).
Norby, T. Solid-state protonic conductors: principles, properties, progress and prospects. Solid State Ion. 125, 1–11 (1999).
US Department of Energy in Multi-Year Research, Development and Demonstration Plan Ch. 3.4 (DOE, 2012); http://energy.gov/sites/prod/files/2014/03/f12/fuel_cells.pdf
Geim, A. K. & Grigorieva, I. V. Van der Waals heterostructures. Nature 499, 419–425 (2013).
Helfferich, F. Ion Exchange (McGraw Hill, 1962).
Christenson, H. K. & Thomson, N. H. The nature of the air-cleaved mica surface. Surf. Sci. Rep. 71, 367–390 (2016).
Kogure, T., Morimoto, K., Tamura, K., Sato, H. & Yamagishi, A. XRD and HRTEM evidence for fixation of cesium ions in vermiculite clay. Chem. Lett. 41, 380–382 (2012).
Lee, S. S., Fenter, P., Nagy, K. L. & Sturchio, N. C. Real-time observation of cation exchange kinetics and dynamics at the muscovite-water interface. Nat. Commun. 8, 15826 (2017).
Shao, J.-J., Raidongia, K., Koltonow, A. R. & Huang, J. Self-assembled two-dimensional nanofluidic proton channels with high thermal stability. Nat. Commun. 6, 7602 (2015).
Claesson, P. M. et al. An ESCA and AES study of ion-exchange on the basal plane of mica. J. Colloid Interface Sci. 109, 31–39 (1986).
Alcantar, N., Israelachvili, J. & Boles, J. Forces and ionic transport between mica surfaces: implications for pressure solution. Geochim. Cosmochim. Acta 67, 1289–1304 (2003).
Xu, L. & Salmeron, M. An XPS and scanning polarization force microscopy study of the exchange and mobility of surface ions on mica. Langmuir 14, 5841–5844 (1998).
Cheng, L., Fenter, P., Nagy, K. L., Schlegel, M. L. & Sturchio, N. C. Molecular-scale density oscillations in water adjacent to a mica surface. Phys. Rev. Lett. 87, 156103–156103 (2001).
Lee, S. S., Fenter, P., Nagy, K. L. & Sturchio, N. C. Monovalent ion adsorption at the muscovite (001)-solution interface: relationships among ion coverage and speciation, interfacial water structure, and substrate relaxation. Langmuir 28, 8637–8650 (2012).
Fukuma, T., Ueda, Y., Yoshioka, S. & Asakawa, H. Atomic-scale distribution of water molecules at the mica-water interface visualized by three-dimensional scanning force microscopy. Phys. Rev. Lett. 104, 016101 (2010).
Ricci, M., Trewby, W., Cafolla, C. & Voïtchovsky, K. Direct observation of the dynamics of single metal ions at the interface with solids in aqueous solutions. Sci. Rep. 7, 43234 (2017).
Bowers, G. M., Bish, D. L. & Kirkpatrick, R. J. Cation exchange at the mineral-water interface: H3O+/K+ competition at the surface of nano-muscovite. Langmuir 24, 10240–10244 (2008).
Sides, P. J., Faruqui, D. & Gellman, A. J. Dynamics of charging of muscovite mica: measurement and modeling. Langmuir 25, 1475–1481 (2009).
Marx, D. Proton transfer 200 years after von Grotthuss: insights from ab initio simulations. ChemPhysChem 7, 1849–1870 (2006).
Kretinin, A. V. et al. Electronic properties of graphene encapsulated with different two-dimensional atomic crystals. Nano Lett. 14, 3270–3276 (2014).
Mauritz, K. & Moore, R. State of understanding of Nafion. Chem. Rev. 104, 4535–4585 (2004).
Candy, J. P., Fouilloux, P. & Renouprez, A. J. Hydrogen adsorption on platinum catalysts: quantitative determination of the various species population. J. Chem. Soc. Faraday Trans. 1, 616–629 (1980).
Meunier, M., Currie, J. F., Wertheimer, M. R. & Yelon, A. Electrical conduction in biotite micas. J. Appl. Phys. 54, 898–905 (1983).
Li, Q., Aili, D., Aage, H., Jens, H. & Jensen, O. High Temperature Polymer Electrolyte Membrane Fuel Cells (Springer, 2016); https://doi.org/10.1007/978-3-319-17082-4
Zhang, Y. et al. Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv. Mater. 29, 1700132 (2017).
Casciola, M., Alberti, G., Sganappa, M. & Narducci, R. On the decay of Nafion proton conductivity at high temperature and relative humidity. J. Power Sources 162, 141–145 (2006).
Simner, S. P. & Stevenson, J. W. Compressive mica seals for SOFC applications. J. Power Sources 102, 310–316 (2001).
Boscoboinik, J. A., Yu, X., Shaikhutdinov, S. & Freund, H. J. Preparation of an ordered ultra-thin aluminosilicate framework composed of hexagonal prisms forming a percolated network. Microporous Mesoporous Mater. 189, 91–96 (2014).
Bae, S. et al. 30 inch roll-based production of high-quality graphene films for flexible transparent electrodes. Nat. Nanotechnol. 5, 574–578 (2010).
Yang, Q. et al. Ultrathin graphene-based membrane with precise molecular sieving and ultrafast solvent permeation. Nat. Mater. 16, 1198–1202 (2017).
Acknowledgements
The work was supported by the Lloyd’s Register Foundation, the Engineering and Physical Sciences Research Council (EPSRC)—EP/N010345/1, EP/M010619/1 and EP/P009050/1, the European Research Council, the Graphene Flagship and the Royal Society. M.L.-H. acknowledges a Leverhulme Early Career Fellowship, G.-P.H. acknowledges a Marie Curie International Incoming Fellowship, and L.M. acknowledges the EPSRC NOWNano programme for funding. Y.Z. acknowledges the assistance of Eric Prestat in TEM specimen preparation. Computational resources were provided by the TUBITAK ULAKBIM High Performance and Grid Computing Center (TR-Grid e-Infrastructure).
Author information
Authors and Affiliations
Contributions
M.L.-H. and A.K.G. designed and directed the project. L.M. and G.-P.H. fabricated devices, performed measurements and carried out data analysis with help from S.Z. C.B. and F.M.P. provided theoretical support. Y.Z. and S.J.H. performed electron microscopy imaging and analysis. L.M., A.K.G. and M.L.-H. wrote the manuscript. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Nanotechnology thanks Sang Soo Lee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
supplementary Information
Supplementary Figs. 1–8, methods, discussion and refs. 1–37.
Rights and permissions
About this article
Cite this article
Mogg, L., Hao, GP., Zhang, S. et al. Atomically thin micas as proton-conducting membranes. Nat. Nanotechnol. 14, 962–966 (2019). https://doi.org/10.1038/s41565-019-0536-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0536-5
This article is cited by
-
Proton and molecular permeation through the basal plane of monolayer graphene oxide
Nature Communications (2023)
-
Light-enhanced osmotic energy generation with an aramid nanofiber membrane
NPG Asia Materials (2023)
-
Ion exchange in atomically thin clays and micas
Nature Materials (2021)
-
Revisiting Chlor-Alkali Electrolyzers: from Materials to Devices
Transactions of Tianjin University (2021)