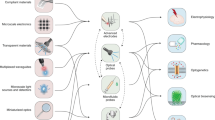
Abstract
Advances in microscopy and molecular strategies have allowed researchers to gain insight into the intricate organization of the mammalian brain and the roles that neurons play in processing information. Despite vast progress, therapeutic strategies for neurological disorders remain limited, owing to a lack of biomaterials for sensing and modulating neuronal signalling in vivo. Therefore, there is a pressing need for developing material-based tools that can form seamless biointerfaces and interrogate the brain with unprecedented resolution. In this Review, we discuss important considerations in material design and implementation, highlight recent breakthroughs in neural sensing and modulation, and propose future directions in neurotechnology research. Our goal is to create an atlas for nano-enabled neural interfaces and to demonstrate how emerging nanotechnologies can interrogate neural systems spanning multiple biological length scales.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Benabid, A. L. Deep brain stimulation for Parkinson's disease. Curr. Opin. Neurobiol. 13, 696–706 (2003).
Terem, I. et al. Revealing sub-voxel motions of brain tissue using phase-based amplified MRI (aMRI). Magn. Reson. Med. 80, 2549–2559 (2018).
Salatino, J. W., Ludwig, K. A., Kozai, T. D. Y. & Purcell, E. K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 1, 862–877 (2017).
Insel, T. R., Landis, S. C. & Collins, F. S. Research priorities. The NIH BRAIN Initiative. Science 340, 687–688 (2013).
Amunts, K. et al. The Human Brain Project: creating a European research infrastructure to decode the human brain. Neuron 92, 574–581 (2016).
Poo, M. M. et al. China Brain Project: basic neuroscience, brain diseases, and brain-inspired computing. Neuron 92, 591–596 (2016).
Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 1, 16063 (2016).
Yang, X. et al. Bioinspired neuron-like electronics. Nat. Mater. 18, 510–517 (2019).
Zhou, T. et al. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc. Natl Acad. Sci. USA 114, 5894–5899 (2017).
Phillips, R. & Quake, S. R. The biological frontier of physics. Phys. Today 59, 38–43 (2006).
Zhang, X. et al. Two-dimensional MoS2-enabled flexible rectenna for Wi-Fi-band wireless energy harvesting. Nature 566, 368–372 (2019).
Shi, Z., Graber, Z. T., Baumgart, T., Stone, H. A. & Cohen, A. E. Cell membranes resist flow. Cell 175, 1769–1779 (2018).
Huberman, A. D., Murray, K. D., Warland, D. K., Feldheim, D. A. & Chapman, B. Ephrin-As mediate targeting of eye-specific projections to the lateral geniculate nucleus. Nat. Neurosci. 8, 1013–1021 (2005).
Dhande, O. S. et al. Development of single retinofugal axon arbors in normal and beta2 knock-out mice. J. Neurosci 31, 3384–3399 (2011).
Hoon, M., Okawa, H., Della Santina, L. & Wong, R. O. Functional architecture of the retina: development and disease. Prog. Retin. Eye Res. 42, 44–84 (2014).
Choi, C. et al. Human eye-inspired soft optoelectronic device using high-density MoS2-graphene curved image sensor array. Nat. Commun. 8, 1664 (2017).
Tang, J. et al. Nanowire arrays restore vision in blind mice. Nat. Commun. 9, 786 (2018).
Dai, X., Hong, G., Gao, T. & Lieber, C. M. Mesh nanoelectronics: seamless integration of electronics with tissues. Acc. Chem. Res. 51, 309–318 (2018).
Ma, Y. et al. Mammalian near-infrared image vision through injectable and self-powered retinal nanoantennae. Cell 177, 243–255 (2019).
Millet, L. J. & Gillette, M. U. Over a century of neuron culture: from the hanging drop to microfluidic devices. Yale J. Biol. Med. 85, 501–521 (2012).
Seabrook, T. A., Burbridge, T. J., Crair, M. C. & Huberman, A. D. Architecture, function, and assembly of the mouse visual system. Annu. Rev. Neurosci. 40, 499–538 (2017).
Park, J. W., Vahidi, B., Taylor, A. M., Rhee, S. W. & Jeon, N. L. Microfluidic culture platform for neuroscience research. Nat. Protoc. 1, 2128–2136 (2006).
Park, J., Koito, H., Li, J. & Han, A. Microfluidic compartmentalized co-culture platform for CNS axon myelination research. Biomed. Microdevices 11, 1145–1153 (2009).
Quadrato, G. et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 (2017).
Sloan, S. A., Andersen, J., Pasca, A. M., Birey, F. & Pasca, S. P. Generation and assembly of human brain region-specific three-dimensional cultures. Nat. Protoc. 13, 2062–2085 (2018).
Pasca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Birey, F. et al. Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59 (2017).
Kato-Negishi, M., Morimoto, Y., Onoe, H. & Takeuchi, S. Millimeter-sized neural building blocks for 3D heterogeneous neural network assembly. Adv. Healthc. Mater. 2, 1564–1570 (2013).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011).
Lancaster, M. A. et al. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 35, 659–666 (2017).
Jiang, Y. W. & Tian, B. Z. Inorganic semiconductor biointerfaces. Nat. Rev. Mater. 3, 473–490 (2018).
Fu, T. M. et al. Sub-10-nm intracellular bioelectronic probes from nanowire–nanotube heterostructures. Proc. Natl Acad. Sci. USA 111, 1259–1264 (2014).
Mirza, M. M. et al. One dimensional transport in silicon nanowire junction-less field effect transistors. Sci. Rep. 7, 3004 (2017).
Colinge, J.-P. et al. Nanowire transistors without junctions. Nat. Nanotechnol. 5, 225–229 (2010).
Tian, B. et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329, 830–834 (2010).
Zhao, Y. et al. Shape-controlled deterministic assembly of nanowires. Nano Lett. 16, 2644–2650 (2016).
Duan, X. et al. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nat. Nanotechnol. 7, 174–179 (2011).
Gao, R. X. et al. Outside looking in: nanotube transistor intracellular sensors. Nano Lett. 12, 3329–3333 (2012).
Cohen-Karni, T. et al. Synthetically encoded ultrashort-channel nanowire transistors for fast, pointlike cellular signal detection. Nano Lett 12, 2639–2644 (2012).
Kang, S. K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Jiang, Y. et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nat. Mater. 15, 1023–1030 (2016).
Park, D. W. et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 5, 5258 (2014).
Kuzum, D. et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 5, 5259 (2014).
Tian, B. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 11, 986–994 (2012).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Xie, C. et al. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 14, 1286–1292 (2015).
Xu, J. et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 355, 59–64 (2017).
Fu, T. M., Hong, G., Viveros, R. D., Zhou, T. & Lieber, C. M. Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proc. Natl Acad. Sci. USA 114, E10046–E10055 (2017).
Koo, J. et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 24, 1830–1836 (2018).
Lee, S. et al. Ultrasoft electronics to monitor dynamically pulsing cardiomyocytes. Nat. Nanotechnol. 14, 156–160 (2019).
Miyamoto, A. et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 12, 907–913 (2017).
Hai, A., Shappir, J. & Spira, M. E. In-cell recordings by extracellular microelectrodes. Nat. Methods 7, 200–202 (2010).
Hai, A. & Spira, M. E. On-chip electroporation, membrane repair dynamics and transient in-cell recordings by arrays of gold mushroom-shaped microelectrodes. Lab Chip 12, 2865–2873 (2012).
Xie, C., Lin, Z., Hanson, L., Cui, Y. & Cui, B. Intracellular recording of action potentials by nanopillar electroporation. Nat. Nanotechnol. 7, 185–190 (2012).
Lin, Z. C., Xie, C., Osakada, Y., Cui, Y. & Cui, B. Iridium oxide nanotube electrodes for sensitive and prolonged intracellular measurement of action potentials. Nat. Commun. 5, 3206 (2014).
Robinson, J. T. et al. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. Nat. Nanotechnol. 7, 180–184 (2012).
Dipalo, M. et al. Intracellular and extracellular recording of spontaneous action potentials in mammalian neurons and cardiac cells with 3D plasmonic nanoelectrodes. Nano Lett. 17, 3932–3939 (2017).
Dipalo, M. et al. Plasmonic meta-electrodes allow intracellular recordings at network level on high-density CMOS-multi-electrode arrays. Nat. Nanotechnol. 13, 965–971 (2018).
Luan, L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Science Advances 3, e1601966 (2017).
Gonzales, D. L. et al. Scalable electrophysiology in intact small animals with nanoscale suspended electrode arrays. Nat. Nanotechnol. 12, 684–691 (2017).
Saha, S., Prakash, V., Halder, S., Chakraborty, K. & Krishnan, Y. A pH-independent DNA nanodevice for quantifying chloride transport in organelles of living cells. Nat. Nanotechnol. 10, 645–651 (2015).
Thubagere, A. J. et al. A cargo-sorting DNA robot. Science 357, eaan6558 (2017).
Bhatia, D. et al. Quantum dot-loaded monofunctionalized DNA icosahedra for single-particle tracking of endocytic pathways. Nat. Nanotechnol. 11, 1112–1119 (2016).
Prakash, V., Saha, S., Chakraborty, K. & Krishnan, Y. Rational design of a quantitative, pH-insensitive, nucleic acid based fluorescent chloride reporter. Chem. Sci. 7, 1946–1953 (2016).
Leung, K., Chakraborty, K., Saminathan, A. & Krishnan, Y. A DNA nanomachine chemically resolves lysosomes in live cells. Nat. Nanotechnol. 14, 176–183 (2019).
Veetil, A. T. et al. Cell-targetable DNA nanocapsules for spatiotemporal release of caged bioactive small molecules. Nat. Nanotechnol. 12, 1183–1189 (2017).
Edelbrock, A. N. et al. Supramolecular nanostructure activates TrkB receptor signaling of neuronal cells by mimicking brain-derived neurotrophic factor. Nano Lett. 18, 6237–6247 (2018).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Shapiro, M. G. et al. Biogenic gas nanostructures as ultrasonic molecular reporters. Nat. Nanotechnol. 9, 311–316 (2014).
Sytnyk, M. et al. Cellular interfaces with hydrogen-bonded organic semiconductor hierarchical nanocrystals. Nat. Commun. 8, 91 (2017).
Tortiglione, C. et al. Semiconducting polymers are light nanotransducers in eyeless animals. Sci. Adv. 3, e1601699 (2017).
Berna, J. et al. Macroscopic transport by synthetic molecular machines. Nat. Mater. 4, 704–710 (2005).
Garcia-Lopez, V. et al. Molecular machines open cell membranes. Nature 548, 567–572 (2017).
Carvalho-de-Souza, J. L., Pinto, B. I., Pepperberg, D. R. & Bezanilla, F. Optocapacitive generation of action potentials by microsecond laser pulses ofnanojoule energy. Biophys. J. 114, 283–288 (2018).
Kubanek, J., Shukla, P., Das, A., Baccus, S. A. & Goodman, M. B. Ultrasound elicits behavioral responses through mechanical effects on neurons and ion channels in a simple nervous system. J. Neurosci. 38, 3081–3091 (2018).
Hallett, M. Transcranial magnetic stimulation and the human brain. Nature 406, 147–150 (2000).
Carvalho-de-Souza, J. L. et al. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 86, 207–217 (2015).
Fang, Y. et al. Texturing silicon nanowires for highly localized optical modulation of cellular dynamics. Nano Lett. 18, 4487–4492 (2018).
Parameswaran, R. et al. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol. 13, 260–266 (2018).
Jiang, Y. W. et al. Rational design of silicon structures for optically controlled multiscale biointerfaces. Nat. Biomed. Eng. 2, 508–521 (2018).
Pliss, A. et al. Subcellular optogenetics enacted by targeted nanotransformers of near-infrared light. ACS Photonics 4, 806–814 (2017).
Haziza, S. et al. Fluorescent nanodiamond tracking reveals intraneuronal transport abnormalities induced by brain-disease-related genetic risk factors. Nat. Nanotechnol. 12, 322–328 (2017).
Huang, H., Delikanli, S., Zeng, H., Ferkey, D. M. & Pralle, A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 5, 602–606 (2010).
Tay, A. & Di Carlo, D. Magnetic nanoparticle-based mechanical stimulation for restoration of mechano-sensitive ion channel equilibrium in neural networks. Nano Lett. 17, 886–892 (2017).
Tay, A., Kunze, A., Murray, C. & Di Carlo, D. Induction of calcium influx in cortical neural networks by nanomagnetic forces. ACS Nano 10, 2331–2341 (2016).
Roet, M. et al. Progress in neuromodulation of the brain: a role for magnetic nanoparticles? Prog. Neurobiol. 177, 1–14 (2019).
Efros, A. L. et al. Evaluating the potential of using quantum dots for monitoring electrical signals in neurons. Nat. Nanotechnol. 13, 278–288 (2018).
Peterka, D. S., Takahashi, H. & Yuste, R. Imaging voltage in neurons. Neuron 69, 9–21 (2011).
Marshall, J. D. & Schnitzer, M. J. Optical strategies for sensing neuronal voltage using quantum dots and other semiconductor nanocrystals. ACS Nano 7, 4601–4609 (2013).
Bonnaud, C. et al. Insertion of nanoparticle clusters into vesicle bilayers. ACS Nano 8, 3451–3460 (2014).
Lee, J. H., Zhang, A., You, S. S. & Lieber, C. M. Spontaneous internalization of cell penetrating peptide-modified nanowires into primary neurons. Nano Lett. 16, 1509–1513 (2016).
Xu, T., Gao, W., Xu, L. P., Zhang, X. & Wang, S. Fuel-free synthetic micro-/nanomachines. Adv. Mater. 29, 1603250 (2017).
Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 (2006).
Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv. Drug Deliv. Rev. 71, 2–14 (2014).
Yoo, S., Hong, S., Choi, Y., Park, J. H. & Nam, Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano 8, 8040–8049 (2014).
Zhao, W. et al. Nanoscale manipulation of membrane curvature for probing endocytosis in live cells. Nat. Nanotechnol. 12, 750–756 (2017).
Tunuguntla, R. H. et al. Bioelectronic light-gated transistors with biologically tunable performance. Adv. Mater. 27, 831–836 (2015).
Chen, S. et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 359, 679–683 (2018).
Zimmerman, J. F. et al. Cellular uptake and dynamics of unlabeled freestanding silicon nanowires. Sci. Adv. 2, e1601039 (2016).
Gu, Y. et al. Rotational dynamics of cargos at pauses during axonal transport. Nat. Commun. 3, 1030 (2012).
Kaplan, L., Ierokomos, A., Chowdary, P., Bryant, Z. & Cui, B. X. Rotation of endosomes demonstrates coordination of molecular motors during axonal transport. Sci. Adv. 4, e1602170 (2018).
Goel, A. & Vogel, V. Harnessing biological motors to engineer systems for nanoscale transport and assembly. Nat. Nanotechnol. 3, 465–475 (2008).
Johannsmeier, S. et al. Gold nanoparticle-mediated laser stimulation induces a complex stress response in neuronal cells. Sci. Rep. 8, 6533 (2018).
Narayanaswamy, N. et al. A pH-correctable, DNA-based fluorescent reporter for organellar calcium. Nat. Methods 16, 95–102 (2019).
Chen, F., Tillberg, P. W. & Boyden, E. S. Optical imaging. Expansion microscopy. Science 347, 543–548 (2015).
Chung, K. & Deisseroth, K. CLARITY for mapping the nervous system. Nat. Methods 10, 508–513 (2013).
Huang, B., Wang, W., Bates, M. & Zhuang, X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, 810–813 (2008).
Denk, W. & Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2, e329 (2004).
Zheng, Z. et al. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 174, 730–743 (2018).
Martersteck, E. M. et al. Diverse central projection patterns of retinal ganglion cells. Cell Rep. 18, 2058–2072 (2017).
Gao, R. et al. Cortical column and whole-brain imaging with molecular contrast and nanoscale resolution. Science 363, eaau8302 (2019).
Beaulieu-Laroche, L. & Harnett, M. T. Dendritic spines prevent synaptic voltage clamp. Neuron 97, 75–82 (2018).
Jayant, K. et al. Targeted intracellular voltage recordings from dendritic spines using quantum-dot-coated nanopipettes. Nat. Nanotechnol. 12, 335–342 (2017).
Patolsky, F. et al. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science 313, 1100–1104 (2006).
Steketee, M. B. et al. Nanoparticle-mediated signaling endosome localization regulates growth cone motility and neurite growth. Proc. Natl Acad. Sci. USA 108, 19042–19047 (2011).
Gautam, V. et al. Engineering highly interconnected neuronal networks on nanowire scaffolds. Nano Lett. 17, 3369–3375 (2017).
Allen, N. J. & Lyons, D. A. Glia as architects of central nervous system formation and function. Science 362, 181–185 (2018).
Deemyad, T., Luthi, J. & Spruston, N. Astrocytes integrate and drive action potential firing in inhibitory subnetworks. Nat. Commun. 9, 4336 (2018).
Kandel, E. R. Principles of Neural Science, 5th edn (McGraw-Hill, 2013).
Nave, K. A. Myelination and support of axonal integrity by glia. Nature 468, 244–252 (2010).
Lee, S. et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922 (2012).
Lee, S., Chong, S. Y. C., Tuck, S. J., Corey, J. M. & Chan, J. R. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nat. Protoc. 8, 771–782 (2013).
Fields, R. D. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat. Rev. Neurosci. 16, 756–767 (2015).
Chen, Y. & Liu, L. H. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 64, 640–665 (2012).
Yang, T. Z. et al. Exosome delivered anticancer drugs across the blood–brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 32, 2003–2014 (2015).
Bonakdar, M., Wasson, E. M., Lee, Y. W. & Davalos, R. V. Electroporation of brain endothelial cells on chip toward permeabilizing the blood–brain barrier. Biophys. J. 110, 503–513 (2016).
Bonakdar, M., Graybill, P. M. & Davalos, R. V. A microfluidic model of the blood–brain barrier to study permeabilization by pulsed electric fields. RSC Adv. 7, 42811–42818 (2017).
Mammadov, B., Mammadov, R., Guler, M. O. & Tekinay, A. B. Cooperative effect of heparan sulfate and laminin mimetic peptide nanofibers on the promotion of neurite outgrowth. Acta Biomater. 8, 2077–2086 (2012).
Tian, B. Z. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 11, 986–994 (2012).
Parameswaran, R. et al. Optical stimulation of cardiac cells with a polymer-supported silicon nanowire matrix. Proc. Natl Acad. Sci. 116, 413–421 (2019).
Hong, G. et al. A method for single-neuron chronic recording from the retina in awake mice. Science 360, 1447–1451 (2018).
Munshi, R. et al. Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice. Elife 6, e27069 (2017).
Chen, R., Romero, G., Christiansen, M. G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 347, 1477–1480 (2015).
Lu, G. J. et al. Acoustically modulated magnetic resonance imaging of gas-filled protein nanostructures. Nat. Mater. 17, 456–463 (2018).
Seo, D. et al. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron 91, 529–539 (2016).
Tian, B. Z. & Lieber, C. M. Nanowired bioelectric interfaces. Chem. Rev. https://doi.org/10.1021/acs.chemrev.8b00795 (2019).
Herisson, F. et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 21, 1209–1217 (2018).
Selberg, J., Gomez, M. & Rolandi, M. The potential for convergence between synthetic biology and bioelectronics. Cell Syst. 7, 231–244 (2018).
Milo, R. & Phillips, R. Cell Biology by the Numbers, 21, 39, 159, 198, 253 (Garland Science, 2016).
Wang, B., Grill, W. M. & Peterchev, A. V. Coupling magnetically induced electric fields to neurons: longitudinal and transverse activation. Biophys. J. 115, 95–107 (2018).
Phillips, M. J. & Voeltz, G. K. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 17, 69–82 (2016).
Millecamps, S. & Julien, J. P. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 14, 161–176 (2013).
Xu, K., Zhong, G. S. & Zhuang, X. W. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456 (2013).
Sherman, D. L. & Brophy, P. J. Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 6, 683–690 (2005).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Duvernoy, H., Delon, S. & Vannson, J. L. The vascularization of the human cerebellar cortex. Brain Res. Bull. 11, 419–480 (1983).
Nicholson, C. & Hrabetova, S. Brain extracellular space: the final frontier of neuroscience. Biophys. J. 113, 2133–2142 (2017).
Budday, S. et al. Mechanical properties of gray and white matter brain tissue by indentation. J. Mech. Behav. Biomed. Mater. 46, 318–330 (2015).
Acknowledgements
B.T. acknowledges support of this work by the Air Force Office of Scientific Research (AFOSR FA9550-18-1-0503), US Army Research Office (W911NF-18-1-0042), US Office of Naval Research (N000141612530, N000141612958) and the National Institutes of Health (NIH NS101488). W.W. acknowledges the National Institutes of Health (1R01NS109990-01). H.A.L. is supported by the National Institutes of Health (F31 EY029156-01A1). F.B. acknowledges the National Institutes of Health (R01-GM030376 and R21-EY027101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Acarón Ledesma, H., Li, X., Carvalho-de-Souza, J.L. et al. An atlas of nano-enabled neural interfaces. Nat. Nanotechnol. 14, 645–657 (2019). https://doi.org/10.1038/s41565-019-0487-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0487-x
This article is cited by
-
Augmenting insect olfaction performance through nano-neuromodulation
Nature Nanotechnology (2024)
-
Why converging technologies need converging international regulation
Ethics and Information Technology (2024)
-
Flexible metallic core–shell nanostructured electrodes for neural interfacing
Scientific Reports (2024)
-
Functional nanoparticle-enabled non-genetic neuromodulation
Journal of Nanobiotechnology (2023)
-
Recent developments in multifunctional neural probes for simultaneous neural recording and modulation
Microsystems & Nanoengineering (2023)