Abstract
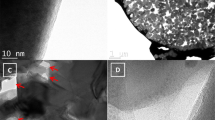
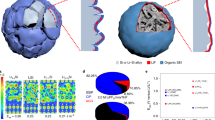
Despite considerable efforts to stabilize lithium metal anode structures and prevent dendrite formation, achieving long cycling life in high-energy batteries under realistic conditions remains extremely difficult due to a combination of complex failure modes that involve accelerated anode degradation and the depletion of electrolyte and lithium metal. Here we report a self-smoothing lithium–carbon anode structure based on mesoporous carbon nanofibres, which, coupled with a lithium nickel–manganese–cobalt oxide cathode with a high nickel content, can lead to a cell-level energy density of 350–380 Wh kg−1 (counting all the active and inactive components) and a stable cycling life up to 200 cycles. These performances are achieved under the realistic conditions required for practical high-energy rechargeable lithium metal batteries: cathode loading ≥4.0 mAh cm−2, negative to positive electrode capacity ratio ≤2 and electrolyte weight to cathode capacity ratio ≤3 g Ah−1. The high stability of our anode is due to the amine functionalization and the mesoporous carbon structures that favour smooth lithium deposition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Grey, C. P. & Tarascon, J. M. Sustainability and in situ monitoring in battery development. Nat. Mater. 16, 45–56 (2017).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Zheng, J. et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2, 17012 (2017).
Lu, Y., Tu, Z. & Archer, L. A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13, 961–969 (2014).
Bouchet, R. Batteries: a stable lithium metal interface. Nat. Nanotechnol. 9, 572–573 (2014).
Liu, Y. et al. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 7, 10992 (2016).
Huang, Z. et al. Protecting the Li-metal anode in a Li–O2 battery by using boric acid as an SEI-forming additive. Adv. Mater. 30, 1803270 (2018).
Lee, S. W. et al. High-power lithium batteries from functionalized carbon-nanotube electrodes. Nat. Nanotechnol. 5, 531–537 (2010).
Li, L. et al. Self-heating-induced healing of lithium dendrites. Science 359, 1513–1516 (2018).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Dudney, N. J. & Li, J. Using all energy in a battery. Science 347, 131–132 (2015).
Han, X. et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572 (2017).
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Choudhury, S., Mangal, R., Agrawal, A. & Archer, L. A. A highly reversible room-temperature lithium metal battery based on crosslinked hairy nanoparticles. Nat. Commun. 6, 10101 (2015).
Yu, D. et al. Scalable synthesis of hierarchically structured carbon nanotube–graphene fibres for capacitive energy storage. Nat. Nanotechnol. 9, 555–562 (2014).
Ye, H. et al. J. Stable Li plating/stripping electrochemistry realized by a hybrid Li reservoir in spherical carbon granules with 3D conducting skeletons. J. Am. Chem. Soc. 139, 5916–5922 (2017).
Liang, Z. et al. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Natl Acad. Sci. USA 113, 2862–2867 (2016).
Zhang, R. et al. Coralloid carbon fiber-based composite lithium anode for robust lithium metal batteries. Joule 2, 764–777 (2018).
Liu, L. et al. Uniform lithium nucleation/growth induced by lightweight nitrogen doped graphitic carbon foams for high performance lithium metal anodes. Adv. Mater. 30, 1706216 (2018).
Ye, H., Xin, S., Yin, Y. X. & Guo, Y. G. Advanced porous carbon materials for high-efficient lithium metal anodes. Adv. Energy Mater. 7, 1700530 (2017).
Wang, T. et al. Ultrafast charging high capacity asphalt–lithium metal batteries. ACS Nano 11, 10761–10767 (2017).
Liu, Y. M. et al. Oxygen and nitrogen co-doped porous carbon granules enabling dendrite-free lithium metal anode. Energy Storage Mater. 18, 320–327 (2019).
Zhang, H., Yu, X. & Braun, P. V. Three-dimensional bicontinuous ultrafast-charge and -discharge bulk battery electrodes. Nat. Nanotechnol. 6, 277–281 (2011).
Huang, S., Tang, L., Najafabadi, H. S., Chen, S. & Ren, Z. A highly flexible semi-tubular carbon film for stable lithium metal anodes in high-performance batteries. Nano Energy 38, 504–509 (2017).
Zhang, Y. et al. High-capacity, low-tortuosity, and channel-guided lithium metal anode. Proc. Natl Acad. Sci. USA 114, 3584–3589 (2017).
Matsuda, S., Kubo, Y., Uosaki, K. & Nakanishi, S. Lithium-metal deposition/dissolution within internal space of CNT 3D matrix results in prolonged cycle of lithium-metal negative electrode. Carbon 119, 119–123 (2017).
Lin, D. et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 11, 626–632 (2016).
Raji, A. R. O. et al. Lithium batteries with nearly maximum metal storage. ACS Nano 11, 6362–6369 (2017).
Zhang, H. et al. Lithiophilic–lithiophobic gradient interfacial layer for a highly stable lithium metal anode. Nat. Commun. 9, 3729 (2018).
Fu, K. et al. Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries. Proc. Natl Acad. Sci. USA 113, 7094–7099 (2016).
Yang, C. P., Yin, Y. X., Zhang, S. F., Li, N. W. & Guo, Y. G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 6, 8058 (2015).
Li, W., Li, M., Wang, M., Zeng, L. & Yu, Y. Electrospinning with partially carbonization in air: highly porous carbon nanofibers optimized for high-performance flexible lithium-ion batteries. Nano Energy 13, 693–701 (2015).
Gireaud, L., Grugeon, S., Laruelle, S., Yrieix, B. & Tarascon, J. M. Lithium metal stripping/plating mechanisms studies: a metallurgical approach. Electrochem. Commun. 8, 1639–1649 (2006).
Sano, H., Sakaebe, H., Senoh, H. & Matsumoto, H. Effect of current density on morphology of lithium electrodeposited in ionic liquid-based electrolytes. J. Electrochem. Soc. 161, A1236–A1240 (2014).
Brissot, C., Rosso, M., Chazalviel, J. N. & Lascaud, S. Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 81, 925–929 (1999).
Liu, Y. et al. Making Li-metal electrodes rechargeable by controlling the dendrite growth direction. Nat. Energy 2, 17083 (2017).
Harry, K. J., Hallinan, D. T., Parkinson, D. Y., MacDowell, A. A. & Balsara, N. P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 13, 69–73 (2014).
Chen, K. H. et al. Dead lithium: mass transport effects on voltage, capacity, and failure of lithium metal anodes. J. Mater. Chem. A 5, 11671–11681 (2017).
Jiao, S. H. et al. Behavior of lithium metal anodes under various capacity utilization and high current density in lithium metal batteries. Joule 2, 1–15 (2018).
Wu, B., Lochala, J., Taverne, T. & Xiao, J. The interplay between solid electrolyte interface (SEI) and dendritic lithium growth. Nano Energy 40, 34–41 (2017).
Kushima, A. et al. Liquid cell transmission electron microscopy observation of lithium metal growth and dissolution: root growth, dead lithium and lithium flotsams. Nano Energy 32, 271–279 (2017).
Lu, D. et al. Failure mechanism for fast-charged lithium metal batteries with liquid electrolytes. Adv. Energy Mater. 5, 1400993 (2015).
Kim, M. S. et al. Langmuir–Blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat. Energy 3, 889–898 (2018).
Niu, C. J. et al. General synthesis of complex nanotubes by gradient electrospinning and controlled pyrolysis. Nat. Commun. 6, 7402 (2015).
Otitoju, T. A., Ahmad, A. L. & Ooi, B. S. Superhydrophilic (superwetting) surface: a review on fabrication and application. J. Ind. Eng. Chem. 47, 19–40 (2017).
Drelich, J., Chibowski, E., Meng, D. D. & Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 7, 9804–9828 (2011).
Lopez, J. et al. Effects of polymer coatings on electrodeposited lithium metal. J. Am. Chem. Soc. 140, 11735–11744 (2018).
Zheng, F., Yang, Y. & Chen, Q. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal–organic framework. Nat. Commun. 5, 5261 (2014).
Ichikawa, T., Hanada, N., Isobe, S., Leng, H. & Fujii, H. Mechanism of novel reaction from LiNH2 and LiH to Li2NH and H2 as a promising hydrogen storage system. J. Phys. Chem. B 108, 7887–7892 (2004).
Janot, R., Eymery, J. B. & Tarascon, J. M. Decomposition of LiAl(NH2)4 and reaction with LiH for a possible reversible hydrogen storage. J. Phys. Chem. C 111, 2335–2340 (2007).
Li, Z. et al. Mechanochemistry of lithium nitride under hydrogen gas. Phys. Chem. Chem. Phys. 17, 21927 (2015).
Christenson, H. K. Two-step crystal nucleation via capillary condensation. CrystEngComm 15, 2030–2039 (2013).
Sun, Y. K. et al. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 8, 320 (2009).
Sun, Y. K. et al. Nanostructured high-energy cathode materials for advanced lithium batteries. Nat. Mater. 11, 942 (2012).
Kimura, N. et al. Cycle deterioration analysis of 0.6 Ah-class lithium-ion cells with cell chemistry of LiNi0.6Co0.2Mn0.2O2-based/graphite. J. Power Sources 332, 187–192 (2016).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy (DOE) through the Advanced Battery Materials Research (BMR) program (Battery500 Consortium) under contract no. DE-AC02-05CH11231. The transmission electron microscopy, STEM, SEM, energy-dispersive X-ray diffraction, X-ray diffraction, X-ray photoemission spectroscopy, Raman, Fourier transform infrared spectroscopy and computational calculations were conducted in the William R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the DOE’s Office of Biological and Environmental Research and located at PNNL. PNNL is operated by Battelle for the DOE under contract DE-AC05-76RL01830. We thank D. Zhao of Fudan University for discussion during the exploration to solve Li wetting. We thank H. Lee of PNNL for optimizing the figures.
Author information
Authors and Affiliations
Contributions
J.L. and C.N. conceived the research and designed the experiments. C.N. performed the material synthesis, characterization, electrochemical measurements and analysed the data. J.M., X.W., Z.L. and L.M. performed some experiments in the exploration to solve Li wetting. L.L. and C.W. carried out the in situ STEM experiment. D.M. performed the molecular dynamics simulation. H.P., W.X., J.X. and J.-G.Z. revised the manuscript. C.N. and J.L. wrote the paper with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Nanotechnology thanks James Tour, Karim Zaghib and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Figures 1–10; Supplementary Tables 1 and 2.
Rights and permissions
About this article
Cite this article
Niu, C., Pan, H., Xu, W. et al. Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 14, 594–601 (2019). https://doi.org/10.1038/s41565-019-0427-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0427-9
This article is cited by
-
Hierarchical Li electrochemistry using alloy-type anode for high-energy-density Li metal batteries
Nature Communications (2024)
-
Cation replacement method enables high-performance electrolytes for multivalent metal batteries
Nature Energy (2024)
-
Lithium-induced graphene layer containing Li3P alloy phase to achieve ultra-stable electrode interface for lithium metal anode
Rare Metals (2024)
-
From Liquid to Solid-State Lithium Metal Batteries: Fundamental Issues and Recent Developments
Nano-Micro Letters (2024)
-
Designing an asymmetric ether-like lithium salt to enable fast-cycling high-energy lithium metal batteries
Nature Energy (2023)