Abstract
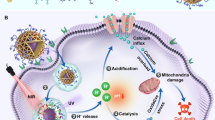
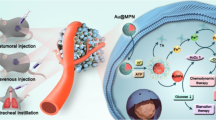
Mitochondrial redox homeostasis, the balance between reactive oxygen species and antioxidants such as glutathione, plays critical roles in many biological processes, including biosynthesis and apoptosis, and thus is a potential target for cancer treatment. Here, we report a mitochondrial oxidative stress amplifier, MitoCAT-g, which consists of carbon-dot-supported atomically dispersed gold (CAT-g) with further surface modifications of triphenylphosphine and cinnamaldehyde. We find that the MitoCAT-g particles specifically target mitochondria and deplete mitochondrial glutathione with atomic economy, thus amplifying the reactive oxygen species damage caused by cinnamaldehyde and finally leading to apoptosis in cancer cells. We show that imaging-guided interventional injection of these particles potently inhibits tumour growth in subcutaneous and orthotopic patient-derived xenograft hepatocellular carcinoma models without adverse effects. Our study demonstrates that MitoCAT-g amplifies the oxidative stress in mitochondria and suppresses tumour growth in vivo, representing a promising agent for anticancer applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data during the study are available from the corresponding authors upon request.
References
Weinberg, S. E. & Chandel, N. S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 11, 9–15 (2015).
Sena, L. A. & Chandel, N. S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012).
Shadel, G. S. & Horvath, T. L. Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569 (2015).
Willems, P. H., Rossignol, R., Dieteren, C. E., Murphy, M. P. & Koopman, W. J. Redox homeostasis and mitochondrial dynamics. Cell. Metab. 22, 207–218 (2015).
Sabharwal, S. S. & Schumacker, P. T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 14, 709–721 (2014).
Dickinson, B. C. & Chang, C. J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 7, 504–511 (2011).
Wallace, D. C. Mitochondria and cancer. Nat. Rev. Cancer 12, 685–698 (2012).
Baulies, A. et al. The 2-oxoglutarate carrier promotes liver cancer by sustaining mitochondrial GSH despite cholesterol loading. Redox Biol. 14, 164–177 (2018).
Gorrini, C., Harris, I. S. & Mak, T. W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12, 931–947 (2013).
Chen, G., Chen, Z., Hu, Y. & Huang, P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by β-phenethyl isothiocyanate: mechanisms for anti-leukemia activity. Antioxid. Redox Sign. 15, 2911–2921 (2011).
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 (2009).
Liu, L. et al. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 16, 132–138 (2017).
Yao, S. et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction. Science 357, 389–393 (2017).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Zhang, Z. et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Nat. Commun. 8, 16100 (2017).
Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Ma, X., Gong, N., Zhong, L., Sun, J. & Liang, X.-J. Future of nanotherapeutics: targeting the cellular sub-organelles. Biomaterials 97, 10–21 (2016).
Zhang, C. J. et al. Mechanism-guided design and synthesis of a mitochondria-targeting artemisinin analogue with enhanced anticancer activity. Angew. Chem. Int. Ed. 128, 13974–13978 (2016).
Ka, H. et al. Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 196, 143–152 (2003).
Grönbeck, H., Curioni, A. & Andreoni, W. Thiols and disulfides on the Au(111) surface: the headgroup–gold interaction. J. Am. Chem. Soc. 122, 3839–3842 (2000).
Chen, F., Li, X., Hihath, J., Huang, Z. & Tao, N. Effect of anchoring groups on single-molecule conductance: comparative study of thiol-, amine-, and carboxylic-acid-terminated molecules. J. Am. Chem. Soc. 128, 15874–15881 (2006).
Miller, J. et al. The effect of gold particle size on Au–Au bond length and reactivity toward oxygen in supported catalysts. J. Catal. 240, 222–234 (2006).
Liu, J. Catalysis by supported single metal atoms. ACS Catal. 7, 34–59 (2016).
Frenkel, A. I., Hills, C. W. & Nuzzo, R. G. A view from the inside: complexity in the atomic scale ordering of supported metal nanoparticles. J. Phys. Chem. B 105, 12689–12703 (2001).
Oberli, L., Monot, R., Mathieu, H., Landolt, D. & Buttet, J. Auger and X-ray photoelectron spectroscopy of small Au particles. Surf. Sci. 106, 301–307 (1981).
Wang, X. et al. Glutathione-triggered ‘off–on’ release of anticancer drugs from dendrimer-encapsulated gold nanoparticles. J. Am. Chem. Soc. 135, 9805–9810 (2013).
Hu, Q., Gao, M., Feng, G. & Liu, B. Mitochondria-targeted cancer therapy using a light-up probe with aggregation-induced-emission characteristics. Angew. Chem. Int. Ed. 53, 14225–14229 (2014).
Han, D. C. et al. 2′-Benzoyloxycinnamaldehyde induces apoptosis in human carcinoma via reactive oxygen species. J. Biol. Chem. 279, 6911–6920 (2004).
Kim, B. et al. Dual acid-responsive micelle-forming anticancer polymers as new anticancer therapeutics. Adv. Funct. Mater. 23, 5091–5097 (2013).
Deng, C., Jiang, Y., Cheng, R., Meng, F. & Zhong, Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: promises, progress and prospects. Nano Today 7, 467–480 (2012).
Shan, X., Jones, D. P., Hashmi, M. & Anders, M. Selective depletion of mitochondrial glutathione concentrations by (R,S)-3-hydroxy-4-pentenoate potentiates oxidative cell death. Chem. Res. Toxicol. 6, 75–81 (1993).
Marí, M. et al. Mechanism of mitochondrial glutathione-dependent hepatocellular susceptibility to TNF despite NF-κB activation. Gastroenterology 134, 1507–1520 (2008).
Esterbauer, H., Schaur, R. J. & Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11, 81–128 (1991).
Hinman, A., Chuang, H.-H., Bautista, D. M. & Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl Acad. Sci. USA 103, 19564–19568 (2006).
Ma, X. et al. Colloidal gold nanoparticles induce changes in cellular and subcellular morphology. ACS Nano 11, 7807–7820 (2017).
Green, D. R. & Reed, J. C. Mitochondria and apoptosis. Science 281, 1309–1311 (1998).
Higuchi, Y. Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem. Pharmacol. 66, 1527–1535 (2003).
Smiley, S. T. et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl Acad. Sci. USA 88, 3671–3675 (1991).
Alavian, K. N. et al. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat. Cell Biol. 13, 1224–1233 (2011).
Schulte, A. & Schuhmann, W. Single-cell microelectrochemistry. Angew. Chem. Int. Ed. 46, 8760–8777 (2007).
Maluccio, M. & Covey, A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 62, 394–399 (2012).
Altekruse, S. F., Henley, S. J., Cucinelli, J. E. & McGlynn, K. A. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am. J. Gastroenterol. 109, 542–553 (2014).
Gao, H. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 21, 1318–1325 (2015).
Lin, S., Lin, C., Lin, C., Hsu, C. & Chen, Y. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 54, 1151–1156 (2005).
Germani, G. et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J. Hepatol. 52, 380–388 (2010).
Livraghi, T. et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology 197, 101–108 (1995).
Zheng, M. et al. Integrating oxaliplatin with highly luminescent carbon dots: an unprecedented theranostic agent for personalized medicine. Adv. Mater. 26, 3554–3560 (2014).
Crooks, R. M., Zhao, M., Sun, L., Chechik, V. & Yeung, L. K. Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 34, 181–190 (2001).
Yuan, L., Lin, W., Xie, Y., Chen, B. & Song, J. Development of a ratiometric fluorescent sensor for ratiometric imaging of endogenously produced nitric oxide in macrophage cells. Chem. Commun. 47, 9372–9374 (2011).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron. Radiat. 12, 537–541 (2005).
Tan, X. et al. The influence of dissolved Si on Ni precipitate formation at the kaolinite water interface: kinetics, DRS and EXAFS analysis. Chemosphere 173, 135–142 (2017).
Price, S. W., Rhodes, J. M., Calvillo, L. & Russell, A. E. Revealing the details of the surface composition of electrochemically prepared Au@Pd core@shell nanoparticles with in situ EXAFS. J. Phys. Chem. C 117, 24858–24865 (2013).
Noh, J. et al. Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat. Commun. 6, 6907 (2015).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (grants nos. 21327806, 21621003, 21235004, 31630027, 31430031, 31600808 and 31225009), the NSFC-German Research Foundation (DFG) project 31761133013 and the ‘Strategic Priority Research Program’ from the Chinese Academy of Sciences (XDA09030301). The authors acknowledge support from the BL14W1 station of the Shanghai Synchrotron Radiation Facility.
Author information
Authors and Affiliations
Contributions
N.G., J.L. and X.-J.L. conceived and designed the experiments. N.G., X.M., X.Y., Q.Z., S.H., T.Z. and S.C. performed the experiments. N.G., X.Y., X.C., X.Tan, S.Y., T.Z., J.Y., H.J., J.L. and X.-J.L. analysed the results. N.G., X.Teng, X.H., Y.G., J.L. and X.-J.L. wrote the manuscript. J.L. and X.-J.L. supervised the entire project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information Nature Nanotechnology thanks Jose Fernandez-Checa, Chun Li and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Figures 1–31, Supplementary Tables 1–2
Rights and permissions
About this article
Cite this article
Gong, N., Ma, X., Ye, X. et al. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat. Nanotechnol. 14, 379–387 (2019). https://doi.org/10.1038/s41565-019-0373-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0373-6
This article is cited by
-
Microbial synthesis of Prussian blue for potentiating checkpoint blockade immunotherapy
Nature Communications (2023)
-
Antioxidant hepatic lipid metabolism can be promoted by orally administered inorganic nanoparticles
Nature Communications (2023)
-
Single-atom catalysts-based catalytic ROS clearance for efficient psoriasis treatment and relapse prevention via restoring ESR1
Nature Communications (2023)
-
Targeting the activity of T cells by membrane surface redox regulation for cancer theranostics
Nature Nanotechnology (2023)
-
Recastable assemblies of carbon dots into mechanically robust macroscopic materials
Nature Communications (2023)