Abstract
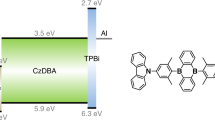
Organic light-emitting transistors are pivotal components for emerging opto- and nanoelectronics applications, such as logic circuitries and smart displays. Within this technology sector, the integration of multiple functionalities in a single electronic device remains the key challenge. Here we show optically switchable organic light-emitting transistors fabricated through a judicious combination of light-emitting semiconductors and photochromic molecules. Irradiation of the solution-processed films at selected wavelengths enables the efficient and reversible tuning of charge transport and electroluminescence simultaneously, with a high degree of modulation (on/off ratios up to 500) in the three primary colours. Different emitting patterns can be written and erased through a non-invasive and mask-free process, on a length scale of a few micrometres in a single device, thereby rendering this technology potentially promising for optically gated highly integrated full-colour displays and active optical memory.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Muccini, M. A bright future for organic field-effect transistors. Nat. Mater. 5, 605–613 (2006).
Cicoira, F. & Santato, C. Organic light emitting field effect transistors: advances and perspectives. Adv. Funct. Mater. 17, 3421–3434 (2007).
Santato, C., Cicoira, F. & Martel, R. Spotlight on organic transistors. Nat. Photon. 5, 392–393 (2011).
Zhang, C., Chen, P. & Hu, W. Organic light-emitting transistors: materials, device configurations, and operations. Small 12, 1252–1294 (2016).
Zaumseil, J. & Sirringhaus, H. Electron and ambipolar transport in organic field-effect transistors. Chem. Rev. 107, 1296–1323 (2007).
Zaumseil, J., Friend, R. H. & Sirringhaus, H. Spatial control of the recombination zone in an ambipolar light-emitting organic transistor. Nat. Mater. 5, 69–74 (2005).
Hsu, B. B. Y. et al. Control of efficiency, brightness, and recombination zone in light-emitting field effect transistors. Adv. Mater. 24, 1171–1175 (2012).
Swensen, J. S., Soci, C. & Heeger, A. J. Light emission from an ambipolar semiconducting polymer field-effect transistor. Appl. Phys. Lett. 87, 253511 (2005).
Bisri, S. Z. et al. High mobility and luminescent efficiency in organic single-crystal light-emitting transistors. Adv. Funct. Mater. 19, 1728–1735 (2009).
Capelli, R. et al. Interface functionalities in multilayer stack organic light emitting transistors (OLETs). Adv. Funct. Mater. 24, 5603–5613 (2014).
Capelli, R. et al. Organic light-emitting transistors with an efficiency that outperforms the equivalent light-emitting diodes. Nat. Mater. 9, 496–503 (2010).
McCarthy, M. A. et al. Low-voltage, low-power, organic light-emitting transistors for active matrix displays. Science 332, 570–573 (2011).
Zaumseil, J., Donley, C. L., Kim, J. S., Friend, R. H. & Sirringhaus, H. Efficient top-gate, ambipolar, light-emitting field-effect transistors based on a green-light-emitting polyfluorene. Adv. Mater. 18, 2708–2712 (2006).
Gwinner, M. C. et al. Highly efficient single-layer polymer ambipolar light-emitting field-effect transistors. Adv. Mater. 24, 2728–2734 (2012).
Ullah, M. et al. Simultaneous enhancement of brightness, efficiency, and switching in RGB organic light emitting transistors. Adv. Mater. 25, 6213–6218 (2013).
Park, S. K. et al. Highly luminescent 2D-type slab crystals based on a molecular charge-transfer complex as promising organic light-emitting transistor materials. Adv. Mater. 29, 1701346 (2017).
Yang, Y., da Costa, R. C., Fuchter, M. J. & Campbell, A. J. Circularly polarized light detection by a chiral organic semiconductor transistor. Nat. Photon. 7, 634–638 (2013).
Kim, Y. L. et al. Voltage-switchable photocurrents in single-walled carbon nanotube–silicon junctions for analog and digital optoelectronics. Nat. Photon. 8, 239–243 (2014).
Zhang, Y. J., Oka, T., Suzuki, R., Ye, J. T. & Iwasa, Y. Electrically switchable chiral light-emitting transistor. Science 344, 725–728 (2014).
Wang, X. et al. A spectrally tunable all-graphene-based flexible field-effect light-emitting device. Nat. Commun. 6, 7767 (2015).
Zhang, X. Y., Hou, L. L. & Samorì, P. Coupling carbon nanomaterials with photochromic molecules for the generation of optically responsive materials. Nat. Commun. 7, 11128 (2016).
Orgiu, E. et al. Optically switchable transistor via energy-level phototuning in a bicomponent organic semiconductor. Nat. Chem. 4, 675–679 (2012).
Gemayel, M. E. et al. Optically switchable transistors by simple incorporation of photochromic systems into small-molecule semiconducting matrices. Nat. Commun. 6, 6330 (2015).
Borjesson, K. et al. Optically switchable transistors comprising a hybrid photochromic molecule/n-type organic active layer. J. Mater. Chem. C 3, 4156–4161 (2015).
Irie, M. & Mohri, M. Thermally irreversible photochromic systems. Reversible photocyclization of diarylethene derivatives. J. Org. Chem. 53, 803–808 (1988).
Irie, M., Fukaminato, T., Matsuda, K. & Kobatake, S. Photochromism of diarylethene molecules and crystals: memories, switches, and actuators. Chem. Rev. 114, 12174–12277 (2014).
Hou, L., Zhang, X., Pijper, T. C., Browne, W. R. & Feringa, B. L. Reversible photochemical control of singlet oxygen generation using diarylethene photochromic switches. J. Am. Chem. Soc. 136, 910–913 (2014).
Leydecker, T. et al. Flexible non-volatile optical memory thin-film transistor device with over 256 distinct levels based on an organic bicomponent blend. Nat. Nanotechnol. 11, 769–775 (2016).
Herder, M. et al. Improving the fatigue resistance of diarylethene switches. J. Am. Chem. Soc. 137, 2738–2747 (2015).
Herder, M. et al. Light-controlled reversible modulation of frontier molecular orbital energy levels in trifluoromethylated diarylethenes. Chem. Eur. J. 23, 3743–3754 (2017).
Salleo, A., Chabinyc, M. L., Yang, M. S. & Street, R. A. Polymer thin-film transistors with chemically modified dielectric interfaces. Appl. Phys. Lett. 81, 4383–4385 (2002).
Ito, Y. et al. Crystalline ultrasmooth self-assembled monolayers of alkylsilanes for organic field-effect transistors. J. Am. Chem. Soc. 131, 9396–9404 (2009).
Lee, T.-W. & Park, O. O. The effect of different heat treatments on the luminescence efficiency of polymer light-emitting diodes. Adv. Mater. 12, 801–804 (2000).
Grell, M., Bradley, D. D. C., Inbasekaran, M. & Woo, E. P. A glass-forming conjugated main-chain liquid crystal polymer for polarized electroluminescence applications. Adv. Mater. 9, 798–802 (1997).
Perevedentsev, A., Chander, N., Kim, J. S. & Bradley, D. D. C. Spectroscopic properties of poly(9,9‐dioctylfluorene) thin films possessing varied fractions of β-phase chain segments: enhanced photoluminescence efficiency via conformation structuring. J. Polym. Sci. Polym. Phys. 54, 1995–2006 (2016).
Caruso, M. E., Lattante, S., Cingolani, R. & Anni, M. Microscopic investigation of the poly(9,9-dioctylfluorene) photoluminescence dependence on the deposition conditions by confocal laser microscopy. Appl. Phys. Lett. 88, 181906 (2006).
Lim, S. F. et al. Suppression of green emission in a new class of blue-emitting PF copolymers with twisted biphenyl moieties. Adv. Funct. Mater. 15, 981–988 (2005).
List, E. J. W., Guentner, R., Scanducci de Freitas, P. & Scherf, U. The effect of keto defect sites on the emission properties of polyfluorene-type materials. Adv. Mater. 14, 374–378 (2002).
Honmou, Y. et al. Single-molecule electroluminescence and photoluminescence of polyfluorene unveils the photophysics behind the green emission band. Nat. Commun. 5, 4666 (2014).
Gong, X. et al. Stabilized blue emission from polyfluorene-based light-emitting diodes: elimination of fluorenone defects. Adv. Funct. Mater. 13, 325–330 (2003).
Hepp, A. et al. Light-emitting field-effect transistor based on a tetracene thin film. Phys. Rev. Lett. 91, 157406 (2003).
Santato, C. et al. Tetracene-based organic light-emitting transistors: optoelectronic properties and electron injection mechanism. Synth. Met. 146, 329–334 (2004).
Roelofs, W. S. C., Adriaans, W. H., Janssen, R. A. J., Kemerink, M. & de Leeuw, D. M. Light emission in the unipolar regime of ambipolar organic field-effect transistors. Adv. Funct. Mater. 23, 4133–4139 (2013).
Redecker, M., Bradley, D. D. C., Inbasekaran, M. & Woo, E. P. Mobility enhancement through homogeneous nematic alignment of a liquid-crystalline polyfluorene. Appl. Phys. Lett. 74, 1400–1402 (1999).
Hsu, B. B. Y. et al. Ordered polymer nanofibers enhance output brightness in bilayer light-emitting field-effect transistors. ACS Nano 7, 2344–2351 (2013).
Lu, H. H., Liu, C. Y., Chang, C. H. & Chen, S. A. Self-dopant formation in poly(9,9-di-n-octylfluorene) via a dipping method for efficient and stable pure-blue electroluminescence. Adv. Mater. 19, 2574–2579 (2007).
Zacharias, P., Gather, M. C., Köhnen, A., Rehmann, N. & Meerholz, K. Photoprogrammable organic light-emitting diodes. Angew. Chem. Int. Ed. 48, 4038–4041 (2009).
Scott, J. C. & Bozano, L. D. Nonvolatile memory elements based on organic materials. Adv. Mater. 19, 1452–1463 (2007).
Acknowledgements
The authors acknowledge funding from the European Commission through the Marie Sklodowska-Curie ITN project iSwitch (GA-642196), the Marie Sklodowska-Curie ITN project SYNCHRONICS (GA-643238), ERC projects SUPRAFUNCTION (GA-257305) and LIGHT4FUNCTION (GA-308117), the Agence Nationale de la Recherche through the Labex project CSC (ANR-10-LABX-0026 CSC) within the Investissement d’Avenir programme (ANR-10-120 IDEX-0002-02), the International Center for Frontier Research in Chemistry (icFRC) as well as the German Research Foundation (via SFB 765 and SFB 951). F.C. is a Royal Society Wolfson Research Merit Award holder.
Author information
Authors and Affiliations
Contributions
L.H., X.Z. and P.S. conceived the experiments. M.H., B.M.S., M.P. and S.H. synthesized the DAEs. L.H. carried out UV–vis absorption and photoluminescence measurements. X.Z. performed atomic force microscopy (the F8/DAE_tBu sample by G.C.) and CV measurements. L.H. and X.Z. designed the devices, performed the electrical experiments and carried out emitting pattern writing. G.F.C., G.C. and F.C. designed and built the device characterization set-up. G.F.C., G.C. and L.H. performed the quantitative OLET device characterization. All authors discussed the results and contributed to interpretation of data. L.H., X.Z. and P.S. co-wrote the paper, with input from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Journal peer review information Nature Nanotechnology thanks Rafaella Capelli and other anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hou, L., Zhang, X., Cotella, G.F. et al. Optically switchable organic light-emitting transistors. Nat. Nanotechnol. 14, 347–353 (2019). https://doi.org/10.1038/s41565-019-0370-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0370-9
This article is cited by
-
Indefinite and bidirectional near-infrared nanocrystal photoswitching
Nature (2023)
-
Efficient energy transfer in organic light-emitting transistor with tunable wavelength
Nano Research (2022)
-
Efficient and low-voltage vertical organic permeable base light-emitting transistors
Nature Materials (2021)
-
Recent advances on π-conjugated polymers as active elements in high performance organic field-effect transistors
Frontiers of Physics (2021)
-
Optical-field driven charge-transfer modulations near composite nanostructures
Nature Communications (2020)