Abstract
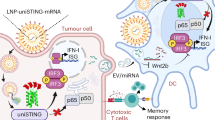
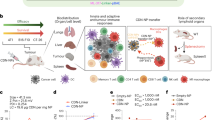
Cyclic dinucleotide (CDN) agonists of stimulator of interferon genes (STING) are a promising class of immunotherapeutics that activate innate immunity to increase tumour immunogenicity. However, the efficacy of CDNs is limited by drug delivery barriers, including poor cellular targeting, rapid clearance and inefficient transport to the cytosol where STING is localized. Here, we describe STING-activating nanoparticles (STING-NPs)—rationally designed polymersomes for enhanced cytosolic delivery of the endogenous CDN ligand for STING, 2′3′ cyclic guanosine monophosphate–adenosine monophosphate (cGAMP). STING-NPs increase the biological potency of cGAMP, enhance STING signalling in the tumour microenvironment and sentinel lymph node, and convert immunosuppressive tumours to immunogenic, tumoricidal microenvironments. This leads to enhanced therapeutic efficacy of cGAMP, inhibition of tumour growth, increased rates of long-term survival, improved response to immune checkpoint blockade and induction of immunological memory that protects against tumour rechallenge. We validate STING-NPs in freshly isolated human melanoma tissue, highlighting their potential to improve clinical outcomes of immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Vanpouille-Box, C. et al. Trial watch: immune checkpoint blockers for cancer therapy. Oncoimmunology 6, e1373237 (2017).
Khalil, D. N., Smith, E. L., Brentjens, R. J. & Wolchok, J. D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 13, 273–290 (2016).
Gotwals, P. et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 17, 286–301 (2017).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Fridman, W. H., Zitvogel, L., Sautes-Fridman, C. & Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14, 717–734 (2017).
Woo, S. R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014).
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Gao, P. et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153, 1094–1107 (2013).
Diner, E. J. et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 3, 1355–1361 (2013).
Ablasser, A. et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384 (2013).
Zhang, X. et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51, 226–235 (2013).
Gao, P. et al. Structure–function analysis of STING activation by c[G(2′,5′)pA(3′,5′)p] and targeting by antiviral DMXAA. Cell 154, 748–762 (2013).
Chen, Q., Sun, L. & Chen, Z. J. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat. Immunol. 17, 1142–1149 (2016).
Corrales, L., McWhirter, S. M., Dubensky, T. W. Jr & Gajewski, T. F. The host STING pathway at the interface of cancer and immunity. J. Clin. Invest. 126, 2404–2411 (2016).
Wang, H. et al. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl Acad. Sci. USA 114, 1637–1642 (2017).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015).
Demaria, O. et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl Acad. Sci. USA 112, 15408–15413 (2015).
Ohkuri, T. et al. Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunol. Immunother. 66, 705–716 (2017).
Curran, E. et al. STING pathway activation stimulates potent immunity against acute myeloid leukemia. Cell Rep. 15, 2357–2366 (2016).
Fu, J. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 7, 283ra252 (2015).
Dubensky, T. W. Jr., Kanne, D. B. & Leong, M. L. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther. Adv. Vaccines 1, 131–143 (2013).
Koshy, S. T., Cheung, A. S., Gu, L., Graveline, A. R. & Mooney, D. J. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv. Biosyst. 1, 1600013 (2017).
Hanson, M. C. et al. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Invest. 125, 2532–2546 (2015).
Mullard, A. Can innate immune system targets turn up the heat on ‘cold’ tumours? Nat. Rev. Drug Discov. 17, 3–5 (2018).
Vrignaud, S., Benoit, J. P. & Saulnier, P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials 32, 8593–8604 (2011).
Manganiello, M. J., Cheng, C., Convertine, A. J., Bryers, J. D. & Stayton, P. S. Diblock copolymers with tunable pH transitions for gene delivery. Biomaterials 33, 2301–2309 (2012).
Wilson, J. T. et al. Enhancement of MHC-I antigen presentation via architectural control of pH-responsive, endosomolytic polymer nanoparticles. AAPS. J. 17, 358–369 (2014).
O’Neil, C. P., Suzuki, T., Demurtas, D., Finka, A. & Hubbell, J. A. A novel method for the encapsulation of biomolecules into polymersomes via direct hydration. Langmuir 25, 9025–9029 (2009).
Kilchrist, K. V., Evans, B. C., Brophy, C. M. & Duvall, C. L. Mechanism of enhanced cellular uptake and cytosolic retention of MK2 inhibitory peptide nano-polyplexes. Cell Mol. Bioeng. 9, 368–381 (2016).
Nelson, C. E. et al. Balancing cationic and hydrophobic content of PEGylated siRNA polyplexes enhances endosome escape, stability, blood circulation time, and bioactivity in vivo. ACS Nano 7, 8870–8880 (2013).
Discher, D. E. & Ahmed, F. Polymersomes. Annu. Rev. Biomed. Eng. 8, 323–341 (2006).
Jain, S. & Bates, F. S. Consequences of nonergodicity in aqueous binary PEO–PB micellar dispersions. Macromolecules 2004, 1511–1523 (2004).
Mai, Y. & Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 41, 5969–5985 (2012).
Parker, B. S., Rautela, J. & Hertzog, P. J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer 16, 131–144 (2016).
Yin, H. et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15, 541–555 (2014).
Wilson, D. R. et al. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine 14, 237–246 (2018).
Harlin, H. et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 69, 3077–3085 (2009).
Munn, D. H. & Mellor, A. L. The tumor-draining lymph node as an immune-privileged site. Immunol. Rev. 213, 146–158 (2006).
Reddy, S. T., Rehor, A., Schmoekel, H. G., Hubbell, J. A. & Swartz, M. A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 112, 26–34 (2006).
Reddy, S. T. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 25, 1159–1164 (2007).
Lizotte, P. H. et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 11, 295–303 (2016).
Mantovani, A., Cassatella, M. A., Costantini, C. & Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 (2011).
Liang, H. et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 8, 1736 (2017).
Shi, L. et al. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin. Cancer Res. 22, 1173–1184 (2016).
Ries, C. H. et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25, 846–859 (2014).
Rudqvist, N. P. et al. Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumor-infiltrating T cells. Cancer Immunol. Res. 6, 139–150 (2018).
Murthy, V., Minehart, J. & Sterman, D. H. Local immunotherapy of cancer: innovative approaches to harnessing tumor-specific immune responses. J. Natl Cancer Inst. 109, djx097 (2017).
Overwijk, W. W. et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 198, 569–580 (2003).
Moynihan, K. D. et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 22, 1402–1410 (2016).
Ghosh, S., Basu, S. & Thayumanavan, S. Simultaneous and reversible functionalization of copolymers for biological applications. Macromolecules 2006, 5595–5597 (2006).
Matini, T. et al. Synthesis and characterization of variable conformation pH responsive block co-polymers for nucleic acid delivery and targeted cell entry. Polym. Chem. 5, 1626–1636 (2014).
Gaffney, B. L., Veliath, E., Zhao, J. & Jones, R. A. One-flask synthesis of c-di-GMP and the [Rp,Rp] and [Rp,Sp] thiophosphate analogues. Org. Lett. 12, 3269–3271 (2010).
Acknowledgements
We thank K. Rock for providing the DC2.4 cells, C. Duvall for use of the gel permeation chromatography equipment and in vivo imaging system, A. Richmond and A. Vilgelm for consultation on flow cytometry protocols, and J. Rhoades and A. Merkel for technical advice on tumour models. We thank the Koch Institute Swanson Biotechnology Center (specifically the Nanotechnology Materials Core Facility) for technical support on the cryogenic electron microscopy, the core facilities of the Vanderbilt Institute of Nanoscale Science and Engineering for use of dynamic light scattering and transmission electron microscope instruments, the VUMC Flow Cytometry Shared Resource (supported by the Vanderbilt-Ingram Cancer Center (VICC) (P30 CA68485) and Vanderbilt Digestive Disease Research Center (DK058404)) for the use of BD three-laser LSRII and BD five-laser LSRFortessa flow cytometers, the Vanderbilt Translational Pathology Shared Resource (supported in part by NCI/NIH Cancer Center Support Grant 5P30 CA684850-19) and the Vanderbilt Technologies for Advanced Genomics. 2′3′-cGAMP was provided by the Vanderbilt Institute of Chemical Biology Chemical Synthesis Core. This research was supported by grants from the National Science Foundation (1554623 to J.T.W.), Alex’s Lemonade Stand Foundation (SID924 to J.T.W.), the National Institutes of Health (K23 CA204726/CA/NCI to D.B.J., R00CA181491 to J.M.B. and 5R35GM119569-03 to M.A.), the VICC (Support Grant P30 CA68485 and VICC Ambassador Discovery Grant to M.A., and VICC-Vanderbilt Center for Immunobiology Pilot Grant to J.T.W.), the Melanoma Research Alliance (503565 to J.T.W.) and Stand Up To Cancer (SU2C) (Innovative Research Grant, grant no. SU2C-AACR-IRG 20-17 to J.T.W.). SU2C is a division of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C.
Author information
Authors and Affiliations
Contributions
D.S. and J.T.W. conceived of and designed the experiments. D.S. performed the majority of the experiments and data analysis. K.W.B. created the ISRE luciferase reporter B16.F10 cells used for longitudinal in vivo experimentation and assisted with tumour therapy studies. P.C. synthesized and characterized 2′3′-cGAMP. D.S.Y. and A.K.R.L.-J. obtained cryogenic transmission electron micrographs of nanoparticles. S.S. synthesized and characterized the PDSMA monomer. M.A. assisted with experimental design and cGAMP characterization. M.K. and D.B.J. provided resected tumour samples from melanoma patients. J.M.B. provided guidance on, and assisted with, the NanoString experiments and analysis of multiplexed gene expression data. D.S. and J.T.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.T.W. and D.S. are inventors on a pending patent related to the technology described in this manuscript. D.B.J. serves on the advisory board for Bristol-Myers Squibb and Merck, and receives research support from Bristol-Myers Squibb that is unrelated to this manuscript. J.M.B. receives research funding from Bristol-Myers Squibb, Genentech and Incyte, receives consulting and expertise testimony compensation from Novartis, and has patents pending concerning the use of HLA-DR as a predictive marker in immunotherapy responses.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Table 1 Supplementary Figures 1–14
Rights and permissions
About this article
Cite this article
Shae, D., Becker, K.W., Christov, P. et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 14, 269–278 (2019). https://doi.org/10.1038/s41565-018-0342-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0342-5