Abstract
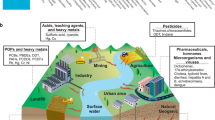
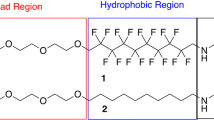
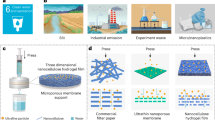
Current technologies for water purification are limited by their contaminant-specific removal capability, requiring multiple processes to meet water quality objectives. Here we show an innovative biomimetic micellar nanocoagulant that imitates the structure of Actinia, a marine predator that uses its tentacles to ensnare food, for the removal of an array of water contaminants with a single treatment step. The Actinia-like micellar nanocoagulant has a core–shell structure and readily disperses in water while maintaining a high stability against aggregation. To achieve effective coagulation, the nanocoagulant everts its configuration, similar to Actinia. The shell hydrolyses into ‘flocs’ and destabilizes and enmeshes colloidal particles while the core is exposed to water, like the extended tentacles of Actinia, and adsorbs the dissolved contaminants. The technology, with its ability to remove a broad spectrum of contaminants and produce high-quality water, has the potential to be a cost-effective replacement for current water treatment processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
23 January 2019
In the version of the Supplementary Information file originally published with this Article, the images used for Supplementary Fig. 4 were incorrect and have now been replaced. This does not affect the results of the Article.
References
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Elimelech, M. The global challenge for adequate and safe water. J. Water Supply Res. Technol. AQUA 55, 3–10 (2006).
Qu, X. L., Alvarez, P. J. J. & Li, Q. L. Applications of nanotechnology in water and wastewater treatment. Water Res. 47, 3931–3946 (2013).
Service, R. F. Desalination freshens up. Science 313, 1088–1090 (2006).
Grant, S. B. et al. Taking the ‘waste’ out of ‘wastewater’ for human water security and ecosystem sustainability. Science 337, 681–686 (2012).
Ali, I. & Gupta, V. K. Advances in water treatment by adsorption technology. Nat. Protoc. 1, 2661–2667 (2006).
Hering, J. G., Waite, T. D., Luthy, R. G., Drewes, J. E. & Sedlak, D. L. A changing framework for urban water systems. Environ. Sci. Technol. 47, 10721–10726 (2013).
Schwarzenbach, R. P. et al. The challenge of micropollutants in aquatic systems. Science 313, 1072–1077 (2006).
Li, J. China gears up to tackle tainted water. Nature 499, 14–15 (2013).
Morel, F. M. M. & Hering, J. G. Principles and Applications of Aquatic Chemistry (Wiley, New York, 1993).
Richardson, S. D. & Temes, T. A. Water analysis: emerging contaminants and current issues. Anal. Chem. 90, 398–428 (2018).
Shon, H. K., Vigneswaran, S. & Snyder, S. A. Effluent organic matter (EfOM) in wastewater: constituents, effects, and treatment. Crit. Rev. Environ. Sci. Technol. 36, 327–374 (2006).
Luo, Y. L. et al. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 473, 619–641 (2014).
Jin, L. Y., Zhang, G. M. & Tian, H. F. Current state of sewage treatment in China. Water Res. 66, 85–98 (2014).
Zodrow, K. R. et al. Advanced materials, technologies, and complex systems analyses: emerging opportunities to enhance urban water security. Environ. Sci. Technol. 51, 10274–10281 (2017).
Westerhoff, P., Alvarez, P., Li, Q. L., Gardea-Torresdey, J. & Zimmerman, J. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci. Nano 3, 1241–1253 (2016).
Mauter, M. S. et al. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 1, 166–175 (2018).
Moreira, F. C., Boaventura, R. A. R., Brillas, E. & Vilar, V. J. P. Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl. Catal. B 202, 217–261 (2017).
Bolisetty, S. & Mezzenga, R. Amyloid–carbon hybrid membranes for universal water purification. Nat. Nanotech. 11, 365–371 (2016).
Werber, J. R., Osuji, C. O. & Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 1, 16018 (2016).
Pintilie, L., Torres, C. M., Teodosiu, C. & Castells, F. Urban wastewater reclamation for industrial reuse: an LCA case study. J. Clean Prod. 139, 1–14 (2016).
Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment (IWA, London, 2006).
Wang, J. P., Yuan, S. J., Wang, Y. & Yu, H. Q. Synthesis, characterization and application of a novel starch-based flocculant with high flocculation and dewatering properties. Water Res. 47, 2643–2648 (2013).
Stumm, W., Morgan, J. J. & Black, A. P. Chemical aspects of coagulation [with discussion]. J. Am. Water Works Assoc. 54, 971–994 (1962).
Stumm, W. & O’Melia, C. R. Stoichiometry of coagulation. J. Am. Water Works Assoc. 60, 514–539 (1968).
Bolong, N., Ismail, A. F., Salim, M. R. & Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 239, 229–246 (2009).
Edzwald, J. K. Coagulation in drinking water treatment: particles, organics and coagulants. Water Sci. Technol. 27, 21–35 (1993).
Krasner, S. W. & Amy, G. Jar-test evaluations of enhanced coagulation. J. Am. Water Works Assoc. 87, 93–107 (1995).
Huang, B. C., Guan, Y. F., Chen, W. & Yu, H. Q. Membrane fouling characteristics and mitigation in a coagulation-assisted microfiltration process for municipal wastewater pretreatment. Water Res. 123, 216–223 (2017).
Fan, X. J. et al. Performance of an integrated process combining ozonation with ceramic membrane ultra-filtration for advanced treatment of drinking water. Desalination 335, 47–54 (2014).
Bolto, B., Dixon, D., Eldridge, R. & King, S. Removal of THM precursors by coagulation or ion exchange. Water Res. 36, 5066–5073 (2002).
Wang, X. M. et al. Preparation and evaluation of titanium-based xerogel as a promising coagulant for water/wastewater treatment. Environ. Sci. Technol. 50, 9619–9626 (2016).
Zeng, Y. & Park, J. Characterization and coagulation performance of a novel inorganic polymer coagulant–poly-zinc-silicate-sulfate. Colloid Surf. A 334, 147–154 (2009).
Tzoupanos, N. D., Zouboulis, A. I. & Tsoleridis, C. A. A systematic study for the characterization of a novel coagulant (polyaluminium silicate chloride). Colloid Surf. A 342, 30–39 (2009).
Haberkamp, J., Ruhl, A. S., Ernst, M. & Jekel, M. Impact of coagulation and adsorption on DOC fractions of secondary effluent and resulting fouling behaviour in ultrafiltration. Water Res. 41, 3794–3802 (2007).
Park, T., Ampunan, V., Lee, S. & Chung, E. Chemical behavior of different species of phosphorus in coagulation. Chemosphere 144, 2264–2269 (2016).
Lacasa, E., Canizares, P., Saez, C., Fernandez, F. J. & Rodrigo, M. A. Removal of nitrates from groundwater by electrocoagulation. Chem. Eng. J. 171, 1012–1017 (2011).
Gabaldon, C. et al. Biological nitrate removal from wastewater of a metal-finishing industry. J. Hazard. Mater. 148, 485–490 (2007).
Suarez, S., Lerna, J. M. & Omil, F. Pre-treatment of hospital wastewater by coagulation—flocculation and flotation. Bioresour. Technol. 100, 2138–2146 (2009).
Liu, J. L. & Wong, M. H. Pharmaceuticals and personal care products (PPCPs): a review on environmental contamination in China. Environ. Int. 59, 208–224 (2013).
Wei, J. C. et al. Comparison of coagulation behavior and floc structure characteristic of different polyferric-cationic polymer dual-coagulants in humic acid solution. Water Res. 43, 724–732 (2009).
Matilainen, A., Vepsalainen, M. & Sillanpaa, M. Natural organic matter removal by coagulation during drinking water treatment: a review. Adv. Colloid Interface Sci. 159, 189–197 (2010).
Wang, S. P. et al. Label-free imaging, detection, and mass measurement of single viruses by surface plasmon resonance. Proc. Natl Acad. Sci. USA 107, 16028–16032 (2010).
Hehre, W. J., Ditchfield, R. & Pople, J. A. Self-consistent molecular-orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular-orbital studies of organic molecules. J. Chem. Phys. 56, 2257–2261 (1972).
Becke, A. D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Mark, P. & Nilsson, L. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. A 105, 9954–9960 (2001).
Jorgensen, W. L., Maxwell, D. S. & TiradoRives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Jiang, W., Yan, T. Y., Wang, Y. T. & Voth, G. A. Molecular dynamics simulation of the energetic room-temperature ionic liquid, 1-hydroxyethyl-4-amino-1,2,4-triazolium nitrate (HEATN). J. Phys. Chem. B 112, 3121–3131 (2008).
Sprenger, K. G., Jaeger, V. W. & Pfaendtner, J. The general AMBER force field (GAFF) can accurately predict thermodynamic and transport properties of many ionic liquids. J. Phys. Chem. B 119, 5882–5895 (2015).
Asensio, J. L., Martinpastor, M. & Jimenezbarbero, J. The use of CVFF and CFF91 force fields in conformational analysis of carbohydrate molecules. Comparison with Amber molecular mechanics and dynamics calculations for methyl α-lactoside. Int. J. Biol. Macromol. 17, 137–148 (1995).
Smith, W. & Forester, T. R. DL_POLY_2.0: a general-purpose parallel molecular dynamics simulation package. J. Mol. Graph. 14, 136–141 (1996).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Acknowledgements
The authors are grateful for financial support from the Major Program of the National Natural Science Foundation of China (grant no. 91434132), the Fund for Innovative Research Group of NSFC (grant no. 51721006) and the US National Science Foundation Graduate Research Fellowship awarded to R.M.D.
Author information
Authors and Affiliations
Contributions
H.Z. conceived the initial idea and experimental design. M.E. and H.Z. supervised the study and experiments. J.L. performed the nanocoagulant synthesis and characterization experiments. N.C. and C.G. investigated the coagulation performance. J.N. and J.L. designed the coagulation behaviour experiments, and R.M.D. and J.L. carried out and analysed the experiments. S.C. carried out the molecular dynamics simulations. C.H., Q.S. and C.X. contributed to the data analysis of the FT-ICR mass spectra. All authors discussed the results and commented on the manuscript. M.E., R.M.D., H.Z. and J.L. wrote the paper with help from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Methods, Supplementary Figures 1–17, Supplementary Tables 1–5
Rights and permissions
About this article
Cite this article
Liu, J., Cheng, S., Cao, N. et al. Actinia-like multifunctional nanocoagulant for single-step removal of water contaminants. Nature Nanotech 14, 64–71 (2019). https://doi.org/10.1038/s41565-018-0307-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0307-8
This article is cited by
-
Carbon-based microelectrodes for environmental remediation: progress, challenges and opportunities
Carbon Letters (2023)
-
Application of Fourier transform ion cyclotron resonance mass spectrometry in molecular characterization of dissolved organic matter
Science China Earth Sciences (2022)
-
Removal of direct dyes by coagulation: Adaptability and mechanism related to the molecular structure
Korean Journal of Chemical Engineering (2022)
-
RETRACTED ARTICLE: Discovery of nanoscale sanal flow choking in cardiovascular system: exact prediction of the 3D boundary-layer-blockage factor in nanotubes
Scientific Reports (2021)
-
Drink safely with biomimetic nanotechnology
Nature Nanotechnology (2019)