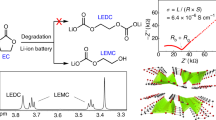
Abstract
The solid–electrolyte interphase (SEI) is probably the least understood component in Li-ion batteries. Considerable effort has been put into understanding its formation and electrochemistry under realistic battery conditions, but mechanistic insights have mostly been inferred indirectly. Here we show the formation of the SEI between a graphite anode and a carbonate electrolyte through combined atomic-scale microscopy and in situ and operando techniques. In particular, we weigh the graphitic anode during its initial lithiation process with an electrochemical quartz crystal microbalance, which unequivocally identifies lithium fluoride and lithium alkylcarbonates as the main chemical components at different potentials. In situ gas analysis confirms the preferential reduction of cyclic over acyclic carbonate molecules, making its reduction product the major component in the SEI. We find that SEI formation starts at graphite edge sites with dimerization of solvated Li+ intercalation between graphite layers. We also show that this lithium salt, at least in its nascent form, can be re-oxidized, despite the general belief that an SEI is electrochemically inert and its formation irreversible.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2017).
Lu, J. et al. The role of nanotechnology in the development of battery materials for electric vehicles. Nat. Nanotech. 11, 1031–1038 (2016).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Winter, M. The solid electrolyte interphase—the most important and the least understood solid electrolyte in rechargeable Li batteries. Z. Phys. Chem. 223, 1395–1406 (2009).
Nitta, N., Wu, F., Lee, J. T. & Yushin, G. Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015).
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2009).
Hu, L. & Xu, K. Nonflammable electrolyte enhances battery safety. Proc. Natl Acad. Sci. USA 111, 3205–3206 (2014).
Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—the solid electrolyte interphase model. J. Electrochem. Soc. 126, 2047–2051 (1979).
An, S. J. et al. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 105, 52–76 (2016).
Peled, E. & Menkin, S. SEI: past, present and future. J. Electrochem. Soc. 164, A1703–A1719 (2017).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4417 (2004).
Lindgren, F. et al. SEI formation and interfacial stability of a Si electrode in a LiTDI-salt based electrolyte with FEC and VC additives for Li-ion batteries. ACS Appl. Mater. Interfaces 8, 15758–15766 (2016).
Andersson, A., Henningson, A., Siegbahn, H., Jansson, U. & Edström, K. Electrochemically lithiated graphite characterised by photoelectron spectroscopy. J. Power Sources 119, 522–527 (2003).
Andersson, A. & Edström, K. Chemical composition and morphology of the elevated temperature SEI on graphite. J. Electrochem. Soc. 148, A1100–A1109 (2001).
Nie, M. et al. Lithium ion battery graphite solid electrolyte interphase revealed by microscopy and spectroscopy. J. Phys. Chem. C 117, 1257–1267 (2013).
Peled, E. et al. Composition, depth profiles and lateral distribution of materials in the SEI built on HOPG-TOF SIMS and XPS studies. J. Power Sources 97, 52–57 (2001).
Domi, Y. et al. Irreversible morphological changes of a graphite negative-electrode at high potentials in LiPF6-based electrolyte solution. Phys. Chem. Chem. Phys. 18, 22426–22433 (2016).
Wang, L., Deng, D., Lev, L. C. & Ng, S. In-situ investigation of solid–electrolyte interphase formation on the anode of Li-ion batteries with atomic force microscopy. J. Power Sources 265, 140–148 (2014).
Zhang, J. et al. Direct observation of inhomogeneous solid electrolyte interphase on MnO anode with atomic force microscopy and spectroscopy. Nano Lett. 12, 2153–2157 (2012).
Yang, T., Sang, L., Ding, F., Zhang, J. & Liu, X. Three- and four-electrode EIS analysis of water stable lithium electrode with solid electrolyte plate. Electrochem. Acta 81, 179–185 (2012).
Huang, J. Y. et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science 330, 1515–1520 (2010).
Zhang, C. et al. Measuring fundamental properties in operating solid oxide electrochemical cells by using in situ X-ray photoelectron spectroscopy. Nat. Mater. 9, 944–949 (2010).
Griffin, J. M. et al. In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors. Nat. Mater. 14, 812–819 (2015).
Shpigel, N. et al. In situ hydrodynamic spectroscopy for structure characterization of porous energy storage electrodes. Nat. Mater. 15, 570–575 (2016).
Song, X. et al. In-situ mass-electrochemical study of surface redox potential and interfacial chemical reactions of Li (Na) FePO4 nanocrystals for Li (Na)-ion batteries. Nano Energy 37, 90–97 (2017).
Jensen, K., Kim, K. & Zettl, A. An atomic-resolution nanomechanical mass sensor. Nat. Nanotech. 3, 533–537 (2008).
Single, F., Horstmann, B. & Latz, A. Revealing SEI morphology: in-depth analysis of a modeling approach. J. Electrochem. Soc. 164, E3132–E3145 (2017).
Single, F., Horstmann, B. & Latz, A. Dynamics and morphology of solid electrolyte interphase (SEI). Phys. Chem. Chem. Phys. 18, 17810–17814 (2016).
Xu, K., Lam, Y., Zhang, S. S., Jow, T. R. & Curtis, T. B. Solvation sheath of Li+ in nonaqueous electrolytes and its implication of graphite/electrolyte interface chemistry. J. Phys. Chem. C 111, 7411–7421 (2007).
Zhuang, G. V., Xu, K., Yang, H., Jow, T. R. & Ross, P. N. Lithium ethylene dicarbonate identified as the primary product of chemical and electrochemical reduction of EC in 1.2 M LiPF6/EC:EMC electrolyte. J. Phys. Chem. B 109, 17567–17573 (2005).
Linstrom, P. J. & Mallard, W. NIST Chemistry Webbook, NIST Standard Reference Database No. 69 (NIST, 2001).
Xu, K. ‘Charge-transfer’ process at graphite/electrolyte interface and the solvation sheath structure of Li+ in nonaqueous electrolytes. J. Electrochem. Soc. 154, A162–A167 (2007).
Dahn, J. R. Phase diagram of LixC6. Phys. Rev. B 44, 9170–9177 (1991).
Wagner, M., Albering, J., Moeller, K.-C., Besenhard, J. & Winter, M. XRD evidence for the electrochemical formation of Li+(PC) yCn- in PC-based electrolytes. Electrochem. Commun. 7, 947–952 (2005).
Cresce, Av, Russell, S. M., Baker, D. R., Gaskell, K. J. & Xu, K. In situ and quantitative characterization of solid electrolyte interphases. Nano Lett. 14, 1405–1412 (2014).
Acknowledgements
This research was supported financially by the National Materials Genome Project (2016YFB0700600), the Guangdong Innovation Team Project (no. 2013N080) and Shenzhen Science and Technology Research Grants (nos. JCYJ20151015162256516, JCYJ20150729111733470 and JCYJ20160226105838578). J.Lu and K.A. acknowledge support from the US Department of Energy under contract no. DE-AC0206CH11357 with the main support provided by the Vehicle Technologies Office, Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy. Support provided by the China Scholarship Council (CSC) during a visit of T.L. to Argonne National Laboratory is acknowledged.
Author information
Authors and Affiliations
Contributions
F.P., K.X., K.A. and T.L. conceived the work and designed the experiments. L.L., L.T. and K.Y. carried out the in situ AFM results. T.L., X.B., Z.C. and J. Lu performed the electrochemical measurements. T.L., J. Liu and M.L. conducted the TEM measurements. K.A., J. Lu, F.P. and K.X. wrote the manuscript, and all authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Sections 1–7
Rights and permissions
About this article
Cite this article
Liu, T., Lin, L., Bi, X. et al. In situ quantification of interphasial chemistry in Li-ion battery. Nature Nanotech 14, 50–56 (2019). https://doi.org/10.1038/s41565-018-0284-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0284-y
This article is cited by
-
Nanoscale one-dimensional close packing of interfacial alkali ions driven by water-mediated attraction
Nature Nanotechnology (2024)
-
Nanoarchitecture factors of solid electrolyte interphase formation via 3D nano-rheology microscopy and surface force-distance spectroscopy
Nature Communications (2023)
-
Revealing the aging process of solid electrolyte interphase on SiOx anode
Nature Communications (2023)
-
Operando characterization and regulation of metal dissolution and redeposition dynamics near battery electrode surface
Nature Nanotechnology (2023)
-
Imaging solid–electrolyte interphase dynamics using operando reflection interference microscopy
Nature Nanotechnology (2023)