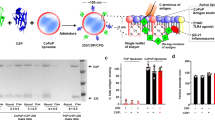
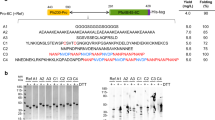
Abstract
Pfs25 is a malaria transmission-blocking vaccine antigen candidate, but its apparently limited immunogenicity in humans has hindered clinical development. Here, we show that recombinant, polyhistidine-tagged (his-tagged) Pfs25 can be mixed at the time of immunization with pre-formed liposomes containing cobalt porphyrin–phospholipid, resulting in spontaneous nanoliposome antigen particleization (SNAP). Antigens are stably presented in uniformly orientated display via his-tag insertion in the cobalt porphyrin–phospholipid bilayer, without covalent modification or disruption of antigen conformation. SNAP immunization of mice and rabbits is well tolerated with minimal local reactogenicity, and results in orders-of-magnitude higher functional antibody generation compared with other ‘mix-and-inject’ adjuvants. Serum-stable antigen binding during transit to draining lymph nodes leads to enhanced antigen uptake by phagocytic antigen-presenting cells, with subsequent generation of long-lived, antigen-specific plasma cells. Seamless multiplexing with four additional his-tagged Plasmodium falciparum polypeptides induces strong and balanced antibody production, illustrating the simplicity of developing multistage particulate vaccines with SNAP immunization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All raw data are available upon request.
References
WHO World Malaria Report 2017 (World Health Organization, 2017).
Nunes, J. K. et al. Development of a transmission-blocking malaria vaccine: progress, challenges, and the path forward. Vaccine 32, 5531–5539 (2014).
Birkett, A. J. Status of vaccine research and development of vaccines for malaria. Vaccine 34, 2915–2920 (2016).
Barr, P. J. et al. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J. Exp. Med. 174, 1203–1208 (1991).
Kaslow, D. C. et al. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333, 74–76 (1988).
Kaslow, D. C. et al. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect. Immun. 62, 5576–5580 (1994).
Kumar, R., Angov, E. & Kumar, N. Potent malaria transmission-blocking antibody responses elicited by Plasmodium falciparum Pfs25 expressed in Escherichia coli after successful protein refolding. Infect. Immun. 82, 1453–1459 (2014).
Gregory, J. A. et al. Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission. PLoS One 7, e37179 (2012).
Mlambo, G., Kumar, N. & Yoshida, S. Functional immunogenicity of baculovirus expressing Pfs25, a human malaria transmission-blocking vaccine candidate antigen. Vaccine 28, 7025–7029 (2010).
Lee, S.-M. et al. Assessment of Pfs25 expressed from multiple soluble expression platforms for use as transmission-blocking vaccine candidates. Malaria J. 15, 405 (2016).
Wu, Y. et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with Montanide ISA 51. PLoS One 3, e2636 (2008).
Malkin, E. M. et al. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 23, 3131–3138 (2005).
Radtke, A. J. et al. Adjuvant and carrier protein-dependent T-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate. Sci. Rep. 7, 40312 (2017).
Shimp, R. L. Jr et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 31, 2954–2962 (2013).
Miyata, T. et al. Plasmodium vivax ookinete surface protein Pvs25 linked to cholera toxin B subunit induces potent transmission-blocking immunity by intranasal as well as subcutaneous immunization. Infect. Immun. 78, 3773–3782 (2010).
Gregory, J. A., Topol, A. B., Doerner, D. Z. & Mayfield, S. Alga-produced cholera toxin-Pfs25 fusion proteins as oral vaccines. Appl. Environ. Microbiol. 79, 3917–3925 (2013).
Kumar, R. et al. Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine 33, 5064–5071 (2015).
Kumar, R. et al. Potent functional immunogenicity of Plasmodium falciparum transmission-blocking antigen (Pfs25) delivered with nanoemulsion and porous polymeric nanoparticles. Pharm. Res. 32, 3827–3836 (2015).
Jones, R. M. et al. A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS One 8, e79538 (2013).
Goodman, A. L. et al. A viral vectored prime-boost immunization regime targeting the malaria Pfs25 antigen induces transmission-blocking activity. PLoS One 6, e29428 (2011).
Li, Y. et al. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci. Rep. 6, 18848 (2016).
Brune, K. D. et al. Plug-and-display: decoration of virus-like particles via isopeptide bonds for modular immunization. Sci. Rep. 6, 19234 (2016).
Shao, S. et al. Functionalization of cobalt porphyrin–phospholipid bilayers with his-tagged ligands and antigens. Nat. Chem. 7, 438–446 (2015).
Lee, S.-M., Plieskatt, J. & King, C. R. Disulfide bond mapping of Pfs25, a recombinant malaria transmission blocking vaccine candidate. Anal. Biochem. 542, 20–23 (2018).
Rüger, R., Müller, D., Fahr, A. & Kontermann, R. E. In vitro characterization of binding and stability of single-chain Fv Ni-NTA-liposomes. J. Drug Target. 14, 576–582 (2006).
Platt, V. et al. Influence of multivalent nitrilotriacetic acid lipid−ligand affinity on the circulation half-life in mice of a liposome-attached His6-protein. Bioconj. Chem. 21, 892–902 (2010).
Bale, S. et al. Covalent linkage of HIV-1 trimers to synthetic liposomes elicits improved B cell and antibody responses. J. Virol. 91, e00443-17 (2017).
Scally, S. W. et al. Molecular definition of multiple sites of antibody inhibition of malaria transmission-blocking vaccine antigen Pfs25. Nat. Commun. 8, 1568 (2017).
Beck, Z. et al. Differential immune responses to HIV-1 envelope protein induced by liposomal adjuvant formulations containing monophosphoryl lipid A with or without QS21. Vaccine 33, 5578–5587 (2015).
Sauer, S. W. & Keim, M. E. Hydroxocobalamin: improved public health readiness for cyanide disasters. Ann. Emerg. Med. 37, 635–641 (2001).
Kuzminski, A. M., Del Giacco, E. J., Allen, R. H., Stabler, S. P. & Lindenbaum, J. Effective treatment of cobalamin deficiency with oral cobalamin. Blood 92, 1191–1198 (1998).
Calabro, S. et al. Vaccine adjuvants Alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29, 1812–1823 (2011).
Liang, F. et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 9, eaal2094 (2017).
Manh, T. P., Alexandre, Y., Baranek, T., Crozat, K. & Dalod, M. Plasmacytoid, conventional, and monocyte-derived dendritic cells undergo a profound and convergent genetic reprogramming during their maturation. Eur. J. Immunol. 43, 1706–1715 (2013).
Ginhoux, F. et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206, 3115–3130 (2009).
Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207, 823–836 (2010).
Rooijen, N. V. & Sanders, A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174, 83–93 (1994).
Allen, T. M., Austin, G. A., Chonn, A., Lin, L. & Lee, K. C. Uptake of liposomes by cultured mouse bone marrow macrophages: influence of liposome composition and size. Biochim. Biophys. Acta 1061, 56–64 (1991).
Fan, Y. C., Sahdev, P., Ochyl, L. J., Akerberg, J. J. & Moon, J. J. Cationic liposome–hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J. Control. Release 208, 121–129 (2015).
Cunningham, A. L. et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N. Engl. J. Med. 375, 1019–1032 (2016).
Viswanathan, S., Rani, C., Vijay Anand, A. & Ho, J. A. Disposable electrochemical immunosensor for carcinoembryonic antigen using ferrocene liposomes and MWCNT screen-printed electrode. Biosens. Bioelectron. 24, 1984–1989 (2009).
Doolan, D. L. & Hoffman, S. L. DNA-based vaccines against malaria: status and promise of the Multi-Stage Malaria DNA Vaccine Operation. Int. J. Parasitol. 31, 753–762 (2001).
Peterson, M. G. et al. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol. Cell. Biol. 9, 3151–3154 (1989).
Lobo, C. A., Konings, R. N. & Kumar, N. Expression of early gametocyte-stage antigens Pfg27 and Pfs16 in synchronized gametocytes and non-gametocyte producing clones of Plasmodium falciparum. Mol. Biochem. Parasitol. 68, 151–154 (1994).
Farrance, C. E. et al. Antibodies to plant-produced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum. Vaccin. 7, 191–198 (2011).
Lee, S. M. et al. An N-terminal Pfs230 domain produced in baculovirus as a biological active transmission-blocking vaccine candidate. Clin. Vaccine Immunol. 24, e00140-17 (2017).
Dutta, S. et al. High antibody titer against apical membrane antigen-1 is required to protect against malaria in the Aotus model. PLoS One 4, e8138 (2009).
Genito, C. J. et al. Liposomes containing monophosphoryl lipid A and QS-21 serve as an effective adjuvant for soluble circumsporozoite protein malaria vaccine FMP013. Vaccine 35, 3865–3874 (2017).
Miura, K. et al. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine 26, 193–200 (2008).
Quakyi, I. A. et al. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J. Immunol. 139, 4213–4217 (1987).
Cheru, L. et al. The IC50 of anti-Pfs25 antibody in membrane-feeding assay varies among species. Vaccine 28, 4423–4429 (2010).
Acknowledgements
This study was supported by PATH’s Malaria Vaccine Initiative, and grants from the National Institutes of Health (R21AI122964 and DP5OD017898) and the intramural programme of the National Institute of Allergy and Infectious Diseases/NIH. The authors acknowledge assistance from G. Mlambo and A. Tripathi with immunofluorescence assays, and input from C. Alving and A. Birkett.
Author information
Authors and Affiliations
Contributions
W.-C.H., C.R.K., S.-M.L. and J.F.L conceived the project. W.-C.H., K.M., S.-M.L. and J.F.L. designed most of the experiments. W.-C.H. and J.F.L. wrote the manuscript. W.-C.H., C.L. and J.G. performed the animal experiments. W.-C.H., C.L., B.D., C.A.L. and K.M. performed and interpreted the ELISA and SMFA experiments. A.R. and J.O. performed transmission electron cryomicroscopy. W.-C.H., K.A.C., C.L., X.H. and B.S. produced and characterized the liposomes. X.H. performed the splenocyte studies. U.C. and W.-C.H. produced fluorescently labelled antigens. J.F. performed the circular dichroism studies. S.D. and S.-M.L. produced the antigens.
Corresponding author
Ethics declarations
Competing interests
W.-C.H., C.R.K., S.-M.L., J.G. and J.F.L are named inventors on a patent application describing this technology. All other authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Methods, Supplementary Figures 1–20 and Supplementary Tables 1–5
Rights and permissions
About this article
Cite this article
Huang, WC., Deng, B., Lin, C. et al. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nature Nanotech 13, 1174–1181 (2018). https://doi.org/10.1038/s41565-018-0271-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0271-3
This article is cited by
-
mRNA vaccines expressing malaria transmission-blocking antigens Pfs25 and Pfs230D1 induce a functional immune response
npj Vaccines (2024)
-
CoPoP liposomes displaying stabilized clade C HIV-1 Env elicit tier 2 multiclade neutralization in rabbits
Nature Communications (2024)
-
Nanotoxicological profiles of clinically approved nanoplatforms
Beni-Suef University Journal of Basic and Applied Sciences (2023)
-
Cationic crosslinked carbon dots-adjuvanted intranasal vaccine induces protective immunity against Omicron-included SARS-CoV-2 variants
Nature Communications (2023)
-
Vaccine co-display of CSP and Pfs230 on liposomes targeting two Plasmodium falciparum differentiation stages
Communications Biology (2022)