Abstract
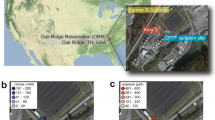
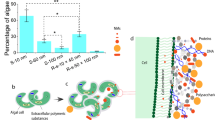
Predicting nanoparticle fate in aquatic environments requires mimicking of ecosystem complexity to observe the geochemical processes affecting their behaviour. Here, 12 nm Au nanoparticles were added weekly to large-scale freshwater wetland mesocosms. After six months, ~70% of Au was associated with the macrophyte Egeria densa, where, despite the thermodynamic stability of Au0 in water, the pristine Au0 nanoparticles were fully oxidized and complexed to cyanide, hydroxyls or thiol ligands. Extracted biofilms growing on E. densa leaves were shown to dissolve Au nanoparticles within days. The Au biodissolution rate was highest for the biofilm with the lowest prevalence of metal-resistant taxa but the highest ability to release cyanide, known to promote Au0 oxidation and complexation. Macrophytes and the associated microbiome thus form a biologically active system that can be a major sink for nanoparticle accumulation and transformations. Nanoparticle biotransformation in these compartments should not be ignored, even for nanoparticles commonly considered to be stable in the environment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schirmer, K. & Auffan, M. Nanotoxicology in the environment. Environ. Sci. Nano 2, 561–563 (2015).
Lowry, G. V. et al. Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland. Environ. Sci. Technol. 46, 7027–7036 (2012).
Auffan, M. et al. An adaptable mesocosm platform for performing integrated assessments of nanomaterial risk in complex environmental systems. Sci. Rep. 4, 5608 (2014).
Stegemeier, J. P., Avellan, A. & Lowry, G. V. Effect of initial speciation of copper- and silver-based nanoparticles on their long-term fate and phytoavailability in freshwater wetland mesocosms. Environ. Sci. Technol. 51, 12114–12122 (2017).
Simonin, M. et al. Engineered nanoparticles interact with nutrients to intensify eutrophication in a wetland ecosystem experiment. Ecol. Appl. https://doi.org/10.1002/eap.1742 (2018).
Auffan, M. et al. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotech. 4, 634–641 (2009).
Khan, I., Saeed, K. & Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. (in the press).
Ferry, J. L. et al. Transfer of gold nanoparticles from the water column to the estuarine food web. Nat. Nanotech. 4, 441–444 (2009).
Burns, J. M. et al. Surface charge controls the fate of Au nanorods in saline estuaries. Environ. Sci. Technol. 47, 12844–12851 (2013).
Glenn, J. B. & Klaine, S. J. Abiotic and biotic factors that influence the bioavailability of gold nanoparticles to aquatic macrophytes. Environ. Sci. Technol. 47, 10223–10230 (2013).
Wray, A. T. & Klaine, S. J. Modeling the influence of physicochemical properties on gold nanoparticle uptake and elimination by Daphnia magna. Environ. Toxicol. Chem. 34, 860–872 (2015).
Lovern, S. B., Owen, H. A. & Klaper, R. Electron microscopy of gold nanoparticle intake in the gut of Daphnia magna. Nanotoxicology 2, 43–48 (2008).
Ferreira, P., Fonte, E., Soares, M. E., Carvalho, F. & Guilhermino, L. Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: gold nanoparticles, microplastics and temperature. Aquat. Toxicol. 170, 89–103 (2016).
Khan, F. R. et al. In vivo retention of ingested Au NPs by Daphnia magna: no evidence for trans-epithelial alimentary uptake. Chemosphere 100, 97–104 (2014).
Mouneyrac, C. et al. Fate and effects of metal-based nanoparticles in two marine invertebrates, the bivalve mollusc Scrobicularia plana and the annelid polychaete Hediste diversicolor. Environ. Sci. Pollut. Res. 21, 7899–7912 (2014).
García-Cambero, J. P. et al. Converging hazard assessment of gold nanoparticles to aquatic organisms. Chemosphere 93, 1194–1200 (2013).
Bar-Ilan, O., Albrecht, R. M., Fako, V. E. & Furgeson, D. Y. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 5, 1897–1910 (2009).
Hull, M. S. et al. Filter-feeding bivalves store and biodeposit colloidally stable gold nanoparticles. Environ. Sci. Technol. 45, 6592–6599 (2011).
Renault, S. et al. Impacts of gold nanoparticle exposure on two freshwater species: a phytoplanktonic alga (Scenedesmus subspicatus) and a benthic bivalve (Corbicula fluminea). Gold Bull. 41, 116–126 (2008).
Lee, B.-T. & Ranville, J. F. The effect of hardness on the stability of citrate-stabilized gold nanoparticles and their uptake by Daphnia magna. J. Hazard. Mater. 213–214, 434–439 (2012).
Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions (Pergamon, New York, NY, 1966).
Reith, F., Lengke, M. F., Falconer, D., Craw, D. & Southam, G. The geomicrobiology of gold. ISME J. 1, 567–584 (2007).
Rea, M. A., Zammit, C. M. & Reith, F. Bacterial biofilms on gold grains—implications for geomicrobial transformations of gold. FEMS Microbiol. Ecol. 92, fiw082 (2016).
Southam, G., Lengke, M. F., Fairbrother, L. & Reith, F. The biogeochemistry of gold. Elements 5, 303–307 (2009).
Unrine, J. M. et al. Evidence for bioavailability of Au nanoparticles from soil and biodistribution within earthworms (Eisenia fetida). Environ. Sci. Technol. 44, 8308–8313 (2010).
Judy, J. D., Unrine, J. M. & Bertsch, P. M. Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environ. Sci. Technol. 45, 776–781 (2011).
Sabo-Attwood, T. et al. Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicology 6, 353–360 (2012).
Dale, A. L. et al. Modeling nanomaterial environmental fate in aquatic systems. Environ. Sci. Technol. 49, 2587–2593 (2015).
Garner, K. L., Suh, S. & Keller, A. A. Assessing the risk of engineered nanomaterials in the environment: development and application of the nanoFate model. Environ. Sci. Technol. 51, 5541–5551 (2017).
Meesters, J. A., Koelmans, A. A., Quik, J. T., Hendriks, A. J. & van de Meent, D. Multimedia modeling of engineered nanoparticles with SimpleBox4nano: model definition and evaluation. Environ. Sci. Technol. 48, 5726–5736 (2014).
Unrine, J. M., Colman, B. P., Bone, A. J., Gondikas, A. P. & Matson, C. W. Biotic and abiotic interactions in aquatic microcosms determine fate and toxicity of Ag nanoparticles. Part 1. Aggregation and dissolution. Environ. Sci. Technol. 46, 6915–6924 (2012).
Schippers, A. & Sand, W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 65, 319–321 (1999).
Aylmore, M. G. & Muir, D. M. Thiosulfate leaching of gold—a review. Miner. Eng. 14, 135–174 (2001).
Dzombak, D. A., Ghosh, R. S. & Wong-Chong, G. M. Cyanide in Water and Soil: Chemistry, Risk, and Management (CRC Press, Boca Raton, FL, 2005).
Reith, F. & McPhail, D. C. Effect of resident microbiota on the solubilization of gold in soil from the Tomakin Park Gold Mine, New South Wales, Australia. Geochim. Cosmochim. Acta 70, 1421–1438 (2006).
Faramarzi, M. A. & Brandl, H. Formation of water-soluble metal cyanide complexes from solid minerals by Pseudomonas plecoglossicida. FEMS Microbiol. Lett. 259, 47–52 (2006).
Brandl, H., Lehmann, S., Faramarzi, M. A. & Martinelli, D. Biomobilization of silver, gold, and platinum from solid waste materials by HCN−forming microorganisms. Hydrometallurgy 94, 14–17 (2008).
Fischer, J. M., Reed-Andersen, T., Klug, J. L. & Chalmers, A. G. Spatial pattern of localized disturbance along a southeastern salt marsh tidal creek. Estuaries Coasts 23, 565–571 (2000).
Dhir, B., Sharmila, P. & Saradhi, P. P. Potential of aquatic macrophytes for removing contaminants from the environment. Crit. Rev. Environ. Sci. Technol. 39, 754–781 (2009).
Sabater, S. et al. Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal. Bioanal. Chem. 387, 1425–1434 (2007).
Baudrimont, M. et al. Trophic transfer and effects of gold nanoparticles (AuNPs) in Gammarus fossarum from contaminated periphytic biofilm. Environ. Sci. Pollut. Res. 25, 11181–11191 (2018).
Colman, B. P. et al. Emerging contaminant or an old toxin in disguise? Silver nanoparticle impacts on ecosystems. Environ. Sci. Technol. 48, 5229–5236 (2014).
Nicol, M. J., Fleming, C. A. & Paul, R. L. in The Extractive Metallurgy of Gold in South Africa (ed Stanley, G. G.) Ch. 15 (South African Institute of Mining and Metallurgy, Johannesburg, 1987).
Glenn, J. B., White, S. A. & Klaine, S. J. Interactions of gold nanoparticles with freshwater aquatic macrophytes are size and species dependent. Environ. Toxicol. Chem. 31, 194–201 (2012).
Marsden, J. & House, I. The Chemistry of Gold Extraction 2nd edn (SME, Littleton, CO, 2006).
Lewis, G. & Shaw, C. F. III Competition of thiols and cyanide for gold (i). Inorg. Chem. 25, 58–62 (1986).
Mishra, A. & Malik, A. Recent advances in microbial metal bioaccumulation. Crit. Rev. Environ. Sci. Technol. 43, 1162–1222 (2013).
Yee, N., Benning, L. G., Phoenix, V. R. & Ferris, F. G. Characterization of metal-cyanobacteria sorption reactions: a combined macroscopic and infrared spectroscopic investigation. Environ. Sci. Technol. 38, 775–782 (2004).
Stolz, J. F., Oremland, R. S., Paster, B. J., Dewhirst, F. E. & Vandamme, P. in Bergey’s Manual of Systematics of Archaea and Bacteria (eds Whitman, W. B. et al.) Ch. Sulfurospirillum (Wiley, New York, NY, 2015).
Zheng, S., Wang, B., Li, Y., Liu, F. & Wang, O. Electrochemically active iron (iii)-reducing bacteria in coastal riverine sediments. J. Basic Microbiol. 57, 1045–1054 (2017).
Milan, M. et al. Microbiota and environmental stress: how pollution affects microbial communities in Manila clams. Aquat. Toxicol. 194, 195–207 (2018).
Ouyang, F., Ji, M., Zhai, H., Dong, Z. & Ye, L. Dynamics of the diversity and structure of the overall and nitrifying microbial community in activated sludge along gradient copper exposures. Appl. Microbiol. Biotechnol. 100, 6881–6892 (2016).
Wasi, S., Tabrez, S. & Ahmad, M. Use of Pseudomonas spp. for the bioremediation of environmental pollutants: a review. Environ. Monit. Assess. 185, 8147–8155 (2013).
Ebbs, S. Biological degradation of cyanide compounds. Curr. Opin. Biotechnol. 15, 231–236 (2004).
Dubey, S. K. & Holmes, D. S. Biological cyanide destruction mediated by microorganisms. World J. Microbiol. Biotechnol. 11, 257–265 (1995).
Yin, Y., Liu, J. & Jiang, G. Sunlight-induced reduction of ionic Ag and Au to metallic nanoparticles by dissolved organic matter. ACS Nano 6, 7910–7919 (2012).
Đurović, M. D., Bugarčić, Ž. D., Heinemann, F. W. & Eldik, Rvan Substitution versus redox reactions of gold(iii) complexes with l-cysteine, l-methionine and glutathione. Dalton Trans. 43, 3911–3921 (2014).
Turkevich, J., Stevenson, P. C. & Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 11, 55–75 (1951).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Minakata, K., Nozawa, H., Gonmori, K., Suzuki, M. & Suzuki, O. Determination of cyanide, in urine and gastric content, by electrospray ionization tandem mass spectrometry after direct flow injection of dicyanogold. Anal. Chim. Acta 651, 81–84 (2009).
Acknowledgements
The authors thank S. M. Anderson and B. P. Colman for their help setting up, monitoring and collecting samples during the experiment, S. Marinakos for providing TEM characterization of the Au-NPs, A. Curinier for insights and B. Perrotta for analyses of Au concentrations on mesocosm biofilms. This work was supported by the National Science Foundation (NSF) and the Environmental Protection Agency (EPA) under NSF Cooperative Agreement EF-0830093 and DBI-1266252, Center for the Environmental Implications of NanoTechnology (CEINT). Funds for graduate student summer support was supplied by the Duke Wetland Center Endowment. Portions of this work were performed at the Stanford Synchrotron Radiation Lightsource (SSRL) on beamline 11-2, a Department of Energy supported facility.
Author information
Authors and Affiliations
Contributions
A.A., M.S., E.M., N.B., E.S.-S. and J.D.R. performed experiments and/or data analysis. A.A., M.S., E.S.B., N.K.G., M.R.W., J.M.U. and G.V.L. were involved in experimental design and writing. All authors discussed the results and commented on the manuscript.
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supporting information
Supplementary Figures 1–7, Supplementary Table 1
Supplementary dataset
Sequence file 1
Supplementary dataset
Sequence file 2
Supplementary dataset
Sequence file 3
Supplementary dataset
Sequence file 4
Rights and permissions
About this article
Cite this article
Avellan, A., Simonin, M., McGivney, E. et al. Gold nanoparticle biodissolution by a freshwater macrophyte and its associated microbiome. Nature Nanotech 13, 1072–1077 (2018). https://doi.org/10.1038/s41565-018-0231-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0231-y
This article is cited by
-
Environmental Carriers for Metal Nanoparticles: Transport, Fate, and Eco-risks
Reviews of Environmental Contamination and Toxicology (2023)
-
Nanocontaminants in soil: emerging concerns and risks
International Journal of Environmental Science and Technology (2022)
-
Dissolution of citrate-stabilized, polyethylene glycol–coated carboxyl and amine-functionalized gold nanoparticles in simulated biological fluids and environmental media
Journal of Nanoparticle Research (2021)
-
Removal of heavy metals and radionuclides from water using nanomaterials: current scenario and future prospects
Environmental Science and Pollution Research (2020)
-
Opportunities and challenges for nanotechnology in the agri-tech revolution
Nature Nanotechnology (2019)