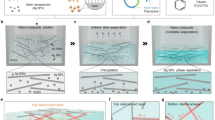
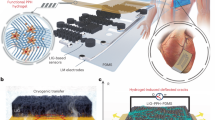
Abstract
Wearable and implantable devices require conductive, stretchable and biocompatible materials. However, obtaining composites that simultaneously fulfil these requirements is challenging due to a trade-off between conductivity and stretchability. Here, we report on Ag–Au nanocomposites composed of ultralong gold-coated silver nanowires in an elastomeric block-copolymer matrix. Owing to the high aspect ratio and percolation network of the Ag–Au nanowires, the nanocomposites exhibit an optimized conductivity of 41,850 S cm−1 (maximum of 72,600 S cm−1). Phase separation in the Ag–Au nanocomposite during the solvent-drying process generates a microstructure that yields an optimized stretchability of 266% (maximum of 840%). The thick gold sheath deposited on the silver nanowire surface prevents oxidation and silver ion leaching, making the composite biocompatible and highly conductive. Using the nanocomposite, we successfully fabricate wearable and implantable soft bioelectronic devices that can be conformally integrated with human skin and swine heart for continuous electrophysiological recording, and electrical and thermal stimulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kim, Y. et al. Stretchable nanoparticle conductors with self-organized conductive pathways. Nature 500, 59–63 (2013).
Park, M. et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotech. 7, 803–809 (2012).
Matsuhisa, N. et al. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater. 16, 834–840 (2017).
Kim, D. H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Son, D. et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotech. 9, 397–404 (2014).
Choi, M. K. et al. Wearable red-green-blue quantum dot light-emitting diode array using high-resolution intaglio transfer printing. Nat. Commun. 6, 7149 (2015).
Lipomi, D. J. et al. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotech. 6, 788–792 (2011).
Miyamoto, A. et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotech. 12, 907–913 (2017).
You, I. et al. Stretchable E-skin apexcardiogram sensor. Adv. Mater. 28, 6359–6364 (2016).
Gong, S. et al. Highly stretchy black gold E-skin nanopatches as highly sensitive wearable biomedical sensors. Adv. Electron. Mater. 1, 1400063 (2015).
Park, J. et al. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci. Transl. Med. 8, 344ra386 (2016).
Lu, C. et al. Flexible and stretchable nanowire-coated fibers for optoelectronic probing of spinal cord circuits. Sci. Adv. 3, e1600955 (2017).
Lee, P. et al. Highly stretchable and highly conductive metal electrode by very long metal nanowire percolation network. Adv. Mater. 24, 3326–3332 (2012).
Choi, S. et al. Stretchable heater using ligand-exchanged silver nanowire nanocomposite for wearable articular thermotherapy. ACS Nano 9, 6626–6633 (2015).
McShan, D., Ray, P. C. & Yu, H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 22, 116–127 (2014).
Yang, M., Hood, Z. D., Yang, X., Chi, M. & Xia, Y. Facile synthesis of Ag@Au core–sheath nanowires with greatly improved stability against oxidation. Chem. Commun. 53, 1965–1968 (2017).
Gong, S. et al. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 5, 3132 (2014).
Chen, Y., Ouyang, Z., Gu, M. & Cheng, W. Mechanically strong, optically transparent, giant metal superlattice nanomembranes from ultrathin gold nanowires. Adv. Mater. 25, 80–85 (2013).
Andres, L. J. et al. Rapid synthesis of ultra-long silver nanowires for tailor-made transparent conductive electrodes: proof of concept in organic solar cells. Nanotechnology 26, 265201 (2015).
Sun, Y., Yin, Y., Mayers, B. T., Herricks, T. & Xia, Y. Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly(vinyl pyrrolidone). Chem. Mater. 14, 4736–4745 (2002).
Liu, H. et al. Etching-free epitaxial growth of gold on silver nanostructures for high chemical stability and plasmonic activity. Adv. Funct. Mater. 25, 5435–5443 (2015).
Yang, Y., Liu, J., Fu, Z. W. & Qin, D. Galvanic replacement-free deposition of Au on Ag for core–shell nanocubes with enhanced chemical stability and SERS activity. J. Am. Chem. Soc. 136, 8153–8156 (2014).
Dong, A. et al. A generalized ligand-exchange strategy enabling sequential surface functionalization of colloidal nanocrystals. J. Am. Chem. Soc. 133, 998–1006 (2011).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotech. 11, 566–572 (2016).
Lee, H., Hong, Y. J., Baik, S., Hyeon, T. & Kim, D. H. Enzyme-based glucose sensor: from invasive to wearable device. Adv. Healthc. Mater. 7, e1701150 (2018).
Li, J. et al. Correlations between percolation threshold, dispersion state, and aspect ratio of carbon nanotubes. Adv. Funct. Mater. 17, 3207–3215 (2007).
Knite, M., Hill, A. J., Pas, S. J., Teteris, V. & Zavickis, J. Effects of plasticizer and strain on the percolation threshold in polyisoprene–carbon nanocomposites: positron annihilation lifetime spectroscopy and electrical resistance measurements. Mater. Sci. Eng. C 26, 771–775 (2006).
Sun, J. Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Dong, J., Abukhdeir, N. M. & Goldthorpe, I. A. Simple assembly of long nanowires through substrate stretching. Nanotechnology 26, 485302 (2015).
Wang, L.-F., Liu, J.-Q., Yang, B. & Yang, C.-S. PDMS-based low cost flexible dry electrode for long-term EEG measurement. IEEE Sens. J. 12, 2898–2904 (2012).
Hurley, M. V. & Bearne, L. M. Non-exercise physical therapies for musculoskeletal conditions. Best Pract. Res. Clin. Rheumatol. 22, 419–433 (2008).
Sarzi-Puttini, P. et al. Osteoarthritis: an overview of the disease and its treatment strategies. Semin. Arthritis Rheum. 35 (Suppl. 1), 1–10 (2005).
Xu, B. et al. An epidermal stimulation and sensing platform for sensorimotor prosthetic control, management of lower back exertion, and electrical muscle activation. Adv. Mater. 28, 4462–4471 (2016).
Lim, S. et al. Transparent and stretchable interactive human machine interface based on patterned graphene heterostructures. Adv. Funct. Mater. 25, 375–383 (2015).
Kuiken, T. A., Marasco, P. D., Lock, B. A., Harden, R. N. & Dewald, J. P. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc. Natl Acad. Sci. USA 104, 20061–20066 (2007).
Lee, S. et al. A strain-absorbing design for tissue–machine interfaces using a tunable adhesive gel. Nat. Commun. 5, 5898 (2014).
Xu, L. et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat. Commun. 5, 3329 (2014).
Lelovas, P. P., Kostomitsopoulos, N. G. & Xanthos, T. T. A comparative anatomic and physiologic overview of the porcine heart. J. Am. Assoc. Lab. Anim. Sci. 53, 432–438 (2014).
Pham, T. & Sun, W. Comparison of biaxial mechanical properties of coronary sinus tissues from porcine, ovine and aged human species. J. Mech. Behav. Biomed. Mater. 6, 21–29 (2012).
Smits, F. M. Measurement of sheet resistivities with the four-point probe. Bell Syst. Tech. J. 37, 711–718 (1958).
Acknowledgements
This work was supported by the Institute for Basic Science (grant numbers IBS-R006-D1 and IBS-R006-A1). The authors thank the staff of the National Center for Inter-university Research Facilities (NCIRF) and the Research Institute of Advanced Materials (RIAM) in Seoul National University. The authors also thank M. Josephson for material and intellectual support of the animal research.
Author information
Authors and Affiliations
Contributions
S.C., S.I.H., D.J., H.J.H., T.H. and D.-H.K. designed the experiments. S.C., S.I.H., D.J., C.L., M.L., H.J.H., T.H. and D.-H.K. performed experiments and analysis. S.C., S.I.H., D.J., H.J.H., C.L., S.B., O.K.P., C.M.T., S.Y.B., S.-W.L., K.P., P.M.K. and R.N. performed in vivo animal experiments and data analysis. S.I.H., S.-W.L. and K.P. performed in vitro experiments and analysis. J.W.Y., J.H.R. and W.B.L. performed computer simulations. S.C., S.I.H., D.J., H.J.H., S.B., T.H. and D.-H.K. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures 1–15, Supplementary References
Supplementary Video
The heat rolling-pressed Ag–Au nanocomposite was stretched to 200%, 400% and 840%
Rights and permissions
About this article
Cite this article
Choi, S., Han, S.I., Jung, D. et al. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nature Nanotech 13, 1048–1056 (2018). https://doi.org/10.1038/s41565-018-0226-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0226-8
This article is cited by
-
Phase-separated stretchable conductive nanocomposite to reduce contact resistance of skin electronics
Scientific Reports (2024)
-
Motion artefact management for soft bioelectronics
Nature Reviews Bioengineering (2024)
-
A Generic Strategy to Create Mechanically Interlocked Nanocomposite/Hydrogel Hybrid Electrodes for Epidermal Electronics
Nano-Micro Letters (2024)
-
Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring
Nano-Micro Letters (2024)
-
Flexible metallic core–shell nanostructured electrodes for neural interfacing
Scientific Reports (2024)