Abstract
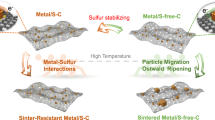
Single noble metal atoms and ultrafine metal clusters catalysts tend to sinter into aggregated particles at elevated temperatures, driven by the decrease of metal surface free energy. Herein, we report an unexpected phenomenon that noble metal nanoparticles (Pd, Pt, Au-NPs) can be transformed to thermally stable single atoms (Pd, Pt, Au-SAs) above 900 °C in an inert atmosphere. The atomic dispersion of metal single atoms was confirmed by aberration-corrected scanning transmission electron microscopy and X-ray absorption fine structures. The dynamic process was recorded by in situ environmental transmission electron microscopy, which showed competing sintering and atomization processes during NP-to-SA conversion. Further, density functional theory calculations revealed that high-temperature NP-to-SA conversion was driven by the formation of the more thermodynamically stable Pd-N4 structure when mobile Pd atoms were captured on the defects of nitrogen-doped carbon. The thermally stable single atoms (Pd-SAs) exhibited even better activity and selectivity than nanoparticles (Pd-NPs) for semi-hydrogenation of acetylene.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zhu, C., Du, D., Eychmüller, A. & Lin, Y. Engineering ordered and nonordered porous noble metal nanostructures: synthesis, assembly, and their applications in electrochemistry. Chem. Rev. 115, 8896–8943 (2015).
Liu, X. et al. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 10, 402–434 (2017).
Furstner, A. Gold and platinum catalysis-a convenient tool for generating molecular complexity. Chem. Soc. Rev. 38, 3208–3221 (2009).
Zhang, S. et al. Catalysis on singly dispersed bimetallic sites. Nat. Commun. 6, 7938 (2015).
Sehested, J., Gelten, J. A. P., Remediakis, I. N., Bengaard, H. & Norskov, J. K. Sintering of nickel steam-reforming catalysts: effects of temperature and steam and hydrogen pressures. J. Catal. 223, 432–443 (2004).
Corma, A. et al. Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity. Nat. Chem. 5, 775–781 (2013).
Xu, Z. et al. Size-dependent catalytic activity of supported metal clusters. Nature 372, 346–348 (1994).
Wang, X., Zhuang, J., Peng, Q. & Li, Y. A general strategy for nanocrystal synthesis. Nature 437, 121–124 (2005).
Wu, Y., Wang, D. & Li, Y. Understanding of the major reactions in solution synthesis of functional nanomaterials. Sci. China Mater. 59, 938–996 (2016).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Thomas, J. M. Catalysis: Tens of thousands of atoms replaced by one. Nature 525, 325–326 (2015).
Fu, Q., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 301, 935–938 (2003).
Hackett, S. F. J. et al. High-activity, single-site mesoporous Pd/Al2O3 catalysts for selective aerobic oxidation of allylic alcohols. Angew. Chem. Int. Ed. 46, 8593–8596 (2007).
Yin, P. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. Int. Ed. 55, 10800–10805 (2016).
Guo, X. et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 344, 616–619 (2014).
Siahrostami, S. et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 12, 1137–1143 (2013).
Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Campbell, C. T., Parker, S. C. & Starr, D. E. The effect of size-dependent nanoparticle energetics on catalyst sintering. Science 298, 811–814 (2002).
Asoro, M. A., Kovar, D., Shao-Horn, Y., Allard, L. F. & Ferreira, P. J. Coalescence and sintering of Pt nanoparticles: in situ observation by aberration-corrected HAADF STEM. Nanotechnology 21, 025701 (2010).
Simonsen, S. B. et al. Direct observations of oxygen-induced platinum nanoparticle ripening studied by in situ TEM. J. Am. Chem. Soc. 132, 7968–7975 (2010).
Hansen, T. W., De La Riva, A. T., Challa, S. R. & Datye, A. K. Sintering of catalytic nanoparticles: particle migration or ostwald ripening? Acc. Chem. Res. 46, 1720–1730 (2013).
Risse, T., Shaikhutdinov, S., Nilius, N., Sterrer, M. & Freund, H.-J. Gold supported on thin oxide films: from single atoms to nanoparticles. Acc. Chem. Res. 41, 949–956 (2008).
Datye, A. K., Xu, Q., Kharas, K. C. & McCarty, J. M. Particle size distributions in heterogeneous catalysts: what do they tell us about the sintering mechanism? Catal. Today 111, 59–67 (2006).
Nagai, Y. et al. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 242, 103–109 (2006).
Li, G. & Jin, R. Atomically precise gold nanoclusters as new model catalysts. Acc. Chem. Res. 46, 1749–1758 (2013).
Zhu, Y., Qian, H., Drake, B. A. & Jin, R. Atomically precise Au25(SR)18 nanoparticles as catalysts for the selective hydrogenation of α,β-unsaturated ketones and aldehydes. Angew. Chem. Int. Ed. 49, 1295–1298 (2010).
Lu, J. et al. Coking- and sintering-resistant palladium catalysts achieved through atomic layer deposition. Science 335, 1205–1208 (2012).
Li, Z. et al. Platinum–nickel frame within metal-organic framework fabricated in situ for hydrogen enrichment and molecular sieving. Nat. Commun. 6, 8248 (2015).
Moliner, M. et al. Reversible transformation of Pt nanoparticles into single atoms inside high-silica chabazite zeolite. J. Am. Chem. Soc. 138, 15743–15750 (2016).
Yang, H., Chen, H., Chen, J., Omotoso, O. & Ring, Z. Shape selective and hydrogen spillover approach in the design of sulfur-tolerant hydrogenation catalysts. J. Catal. 243, 36–42 (2006).
Liu, J.-C., Wang, Y.-G. & Li, J. Toward rational design of oxide-supported single-atom catalysts: atomic dispersion of gold on ceria. J. Am. Chem. Soc. 139, 6190–6199 (2017).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Peterson, E. J. et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 5, 4885 (2014).
Spezzati, G. et al. Atomically dispersed Pd−O species on CeO2(111) as highly active sites for low-temperature CO oxidation. ACS Catal. 7, 6887–6891 (2017).
Chen, Y. et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 56, 6937–6941 (2017).
Buffat, P. & Borel, J. P. Size effect on the melting temperature of gold particles. Phys. Rev. A 13, 2287–2298 (1976).
Lefèvre, M., Proietti, E., Jaouen, F. & Dodelet, J.-P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 324, 71–74 (2009).
Fei., H. et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018).
Campbell, C. T. The energetics of supported metal nanoparticles: relationships to sintering rates and catalytic activity. Acc. Chem. Res. 46, 1712–1719 (2013).
Studt, F. et al. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 320, 1320–1322 (2008).
Xu, B., Zhang, Z. & Wang, X. Formamide: an efficient solvent to synthesize water-soluble and sub-ten-nanometer nanocrystals. Nanoscale 5, 4495–4505 (2013).
Ji, W., Qi, W., H. Tang, S., Peng, H. & Li, S. Hydrothermal synthesis of ultrasmall Pt nanoparticles as highly active electrocatalysts for methanol oxidation. Nanomaterials 5, 2203–2211 (2015).
Chavda, N., Trivedi, A., Thakarda, J., Agrawal, Y. K. & Maity, P. Size specific activity of polymer stabilized gold nanoparticles for transfer hydrogenation catalysis. Catal. Lett. 146, 1331–1339 (2016).
Ziaei-Azad, H. & Semagina, N. Bimetallic catalysts: Requirements for stabilizing PVP removal depend on the surface composition. Appl. Catal. A: Gen. 482, 327–335 (2014).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Ankudinov, A. L., Ravel, B., Rehr, J. J. & Conradson, S. D. Real-space multiple-scattering calculation and interpretation of x-ray-absorption near-edge structure. Phys. Rev. B 58, 7565–7576 (1998).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
This work was supported by China Ministry of Science and Technology under contract 2016YFA (0202801), the National Natural Science Foundation of China (21521091, 21390393, U1463202, 21590792, 91645203), 111 Project (B16028) and the China Postdoctoral Science Foundation (2017M620736). The calculations were performed by using supercomputers at Tsinghua National Laboratory for Information Science and Technology. We thank H. B. Pan, X. S. Zheng and J. F. Zhu from NSRL in Hefei, China for their cooperation on XPS measurements.
Author information
Authors and Affiliations
Contributions
Y.L. and Z.L. conceived the idea and co-wrote the paper. S.W. and Z.L. performed most of the reactions, collected and analysed the data. A.L. and X.H. performed the in situ ETEM characterizations. Y.G., Q.Z. and L.G. performed the aberration-corrected scanning transmission electron microscopy characterizations. J.-C.L., H.X. and J.L. proposed the structural model for the active sites and finished the DFT calculations. W.C., Y.W. and L.Z. carried out the XAFS characterization. The other authors performed some of the experiments, discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–21, Supplementary Tables 1–2 and Supplementary References
Supplementary Video 1
A movie of TEM images showing Pd-NPs transforming to Pd-SAs taken in the temperature window 100 °C to 1,000 °C under an Ar atmosphere.
Rights and permissions
About this article
Cite this article
Wei, S., Li, A., Liu, JC. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nature Nanotech 13, 856–861 (2018). https://doi.org/10.1038/s41565-018-0197-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0197-9
This article is cited by
-
Pt-doped Ru nanoparticles loaded on ‘black gold’ plasmonic nanoreactors as air stable reduction catalysts
Nature Communications (2024)
-
Transient co-tuning of atomic Fe and nanoparticle facets for self-relaying Fenton-like catalysis
Communications Materials (2024)
-
Data-driven rational design of single-atom materials for hydrogen evolution and sensing
Nano Research (2024)
-
Single-atomic activation on ZnIn2S4 basal planes boosts photocatalytic hydrogen evolution
Nano Research (2024)
-
The reformation of catalyst: From a trial-and-error synthesis to rational design
Nano Research (2024)