Abstract
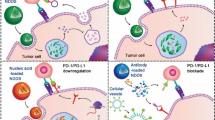
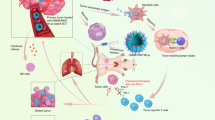
Checkpoint immunotherapy that inhibits tumour immune evasion has demonstrated significant clinical success. However, the therapeutic response is limited to certain patient populations, and immunotoxicity as well as autoimmunity have compromised the therapeutic benefits. Here, we report on an inherently therapeutic fucoidan–dextran-based magnetic nanomedicine (IO@FuDex3) conjugated with a checkpoint inhibitor (anti-PD-L1) and T-cell activators (anti-CD3 and anti-CD28). IO@FuDex3 can repair the immunosuppressive tumour microenvironment by reinvigorating tumour-infiltrating lymphocytes, while targeting the nanomedicine via magnetic navigation to the tumour to minimize off-target effects. Treatment that combines IO@FuDex3 and magnetic navigation reduces the occurrence of adverse events and extends the median survival from 32 to 63 days with less than 1 per cent dose compared with soluble anti-PD-L1. Thus, we demonstrate the potential of integrating anti-PD-L1 and T-cell activators as a form of inherently therapeutic nanomedicine to augment the therapeutic index of combination checkpoint immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hude, I., Sasse, S., Engert, A. & Bröckelmann, P. J. The emerging role of immune checkpoint inhibition in malignant lymphoma. Haematologica 102, 30–42 (2017).
Chen, L. & Han, X. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J. Clin. Invest. 125, 3384–3391 (2015).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Tang, H. et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 29, 285–296 (2016).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Byun, D. J., Wolchok, J. D., Rosenberg, L. M. & Girotra, M. Cancer immunotherapy — immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 13, 195–207 (2017).
June, C. H., Warshauer, J. T., & Bluestone, J. A. Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat. Med. 23, 540–547 (2017).
Mahoney, K. M., Rennert, P. D. & Freeman, G. J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 14, 561–584 (2015).
Carlo, M. I., Voss, M. H. & Motzer, R. J. Checkpoint inhibitors and other novel immunotherapies for advanced renal cell carcinoma. Nat. Rev. Urol. 13, 420–431 (2016).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New Engl. J. Med. 373, 23–34 (2015).
Sun, T. et al. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 53, 12320–12364 (2014).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Fan, Y. & Moon, J. Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines 3, 662–685 (2015).
Allen, T. M. & Cullis, P. R. Drug delivery systems: entering the mainstream. Science 303, 1818–1822 (2004).
Chung, J. E. et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotech 9, 907–912 (2014).
Kwak, J.-Y. Fucoidan as a marine anticancer agent in preclinical development. Mar. Drugs 12, 851–870 (2014).
Hsu, H.-Y. et al. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGFβ receptor degradation in breast cancer. Carcinogenesis 34, 874–884 (2013).
Fitton, J., Stringer, D. & Karpiniec, S. Therapies from fucoidan: an update. Mar. Drugs 13, 5920–5946 (2015).
Jin, J.-O. et al. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific t cell immune responses. PLoS ONE 9, e99396 (2014).
Hermanson, G. T in Bioconjugate Techniques 3rd edn, Ch. 3, 229–258 (Academic Press, Boston, 2013).
Li, Y. & Kurlander, R. J. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: Differing impact on CD8 T cell phenotype and responsiveness to restimulation. J. Transl. Med. 8, 1–15 (2010).
Wang, X. & Rivière, I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol. Ther. Oncolyt. 3, 16015 (2016).
Zhang, H. & Li, X. Interface-mediated fabrication of bowl-like and deflated ballon-like hollow carbon nanospheres. J. Colloid Interface Sci. 452, 141–147 (2015).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941 (2015).
Chiang, C.-S. et al. Synergistic combination of multistage magnetic guidance and optimized ligand density in targeting a nanoplatform for enhanced cancer therapy. Adv. Healthc. Mater. 5, 2131–2141 (2016).
Johnston, A. P. R. et al. Targeting cancer cells: controlling the binding and internalization of antibody-functionalized capsules. ACS Nano 6, 6667–6674 (2012).
Sagiv-Barfi, I. et al. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc. Natl Acad. Sci. USA 112, E966–E972 (2015).
Voltairas, P. A., Fotiadis, D. I. & Michalis, L. K. Hydrodynamics of magnetic drug targeting. J. Biomech. 35, 813–821 (2002).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Villadolid, J. SpringerAmpamp; Amin, A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl. Lung Cancer Res. 4, 560–575 (2015).
Corsello, S. M. et al. Endocrine side effects induced by immune checkpoint inhibitors. J. Clin. Endocrinol. Metab. 98, 1361–1375 (2013).
Nanda, R. et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 34, 2460–2467 (2016).
Goldinger, S. M. et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin. Cancer Res. 22, 4023–4029 (2016).
Francisco, L. M. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 (2009).
Facciabene, A., Motz, G. T. & Coukos, G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 72, 2162–2171 (2012).
Sun, J. et al. Fucoidan inhibits CCL22 production through NF-κB pathway in M2 macrophages: a potential therapeutic strategy for cancer. Sci. Rep. 6, 35855 (2016).
Adeegbe, D. & Nishikawa, H. Natural and induced T regulatory cells in cancer. Front. Immunol. 4, 190 (2013).
Liu, J. et al. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS ONE 6, e19495 (2011).
Schlecker, E. et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol. 189, 5602–5611 (2012).
Tai, C. C. et al. Deep-magnetic-field generator using flexible laminated copper for thermotherapy applications. IEEE Trans. Magn. 50, 1–4 (2014).
Shapiro, B. et al. Shaping magnetic fields to direct therapy to ears and eyes. Annu. Rev. Biomed. Eng. 16, 455–481 (2014).
Lin, R. L. et al. Interpole-type magnetic navigation system for actuation of magnetic drug. Trans. Emerg. Sel. Top. Power Electron. 4, 252–262 (2016).
Sun, Q. et al. Immunotherapy using slow-cycling tumor cells prolonged overall survival of tumor-bearing mice. BMC Med. 10, 172 (2012).
Lübbe, A. S. et al. Clinical experiences with magnetic drug targeting: a phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 56, 4686–4693 (1996).
Bain, C. C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal. Immunol. 6, 498–510 (2013).
Yasuhara, T. et al. Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats. J. Cereb. Blood Flow Metab. 8, 1804–1810 (2008).
Acknowledgements
The authors thank Taiwan’s Ministry of Science and Technology for research grants from: MOST105-2314-B-009-001-MY3; MOST105-2221-E-009-024-MY3; MOST106-2221-E-009-065-MY3; MOST105-2314-B-039-011-MY3; MOST2628-B-039-009-MY3; and China Medical University Hospital (CMU104-S-10, CMU104-S-15-03 and DMR-104-054, DMR-106-070). The authors would like to thank N.-T. Tsou and S.-S. Chien for computer simulation support.
Author information
Authors and Affiliations
Contributions
C.-S.C. conceived the idea and designed the materials. S.-Y.C. designed the project and directed the research. W.-C.S., C.-H.H. and Y.-J.L. contributed to the design of the in vivo studies. Y.-H.L and H.-W.C. performed the materials analysis. C.-S.C., Y.-J.L., C.-H.H., W.-C.S. and R.L. interpreted the data. C.-S.C. wrote the manuscript with the help of S.-Y.C., C.-H.H., W.-C.S. and R.L. All authors discussed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–25, Supplementary Table 1, Supplementary References
Rights and permissions
About this article
Cite this article
Chiang, CS., Lin, YJ., Lee, R. et al. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nature Nanotech 13, 746–754 (2018). https://doi.org/10.1038/s41565-018-0146-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0146-7
This article is cited by
-
Polymer-mediated nanoformulations: a promising strategy for cancer immunotherapy
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Lung cancer immunotherapy: progress, pitfalls, and promises
Molecular Cancer (2023)
-
An updated landscape on nanotechnology-based drug delivery, immunotherapy, vaccinations, imaging, and biomarker detections for cancers: recent trends and future directions with clinical success
Discover Nano (2023)
-
Nano-immunotherapy: overcoming delivery challenge of immune checkpoint therapy
Journal of Nanobiotechnology (2023)
-
CaCO3 powder-mediated biomineralization of antigen nanosponges synergize with PD-1 blockade to potentiate anti-tumor immunity
Journal of Nanobiotechnology (2023)