Abstract
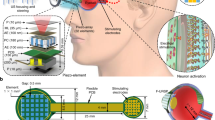
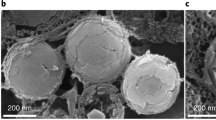
Numerous living organisms possess biophotonic nanostructures that provide colouration and other diverse functions for survival. While such structures have been actively studied and replicated in the laboratory, it remains unclear whether they can be used for biomedical applications. Here, we show a transparent photonic nanostructure inspired by the longtail glasswing butterfly (Chorinea faunus) and demonstrate its use in intraocular pressure (IOP) sensors in vivo. We exploit the phase separation between two immiscible polymers (poly(methyl methacrylate) and polystyrene) to form nanostructured features on top of a Si3N4 substrate. The membrane thus formed shows good angle-independent white-light transmission, strong hydrophilicity and anti-biofouling properties, which prevent adhesion of proteins, bacteria and eukaryotic cells. We then developed a microscale implantable IOP sensor using our photonic membrane as an optomechanical sensing element. Finally, we performed in vivo testing on New Zealand white rabbits, which showed that our device reduces the mean IOP measurement variation compared with conventional rebound tonometry without signs of inflammation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jiang, G. & Zhou, D. D. in Implantable Neural Prostheses 2: Techniques and Engineering Approaches (eds. Zhou, D. & Greenbaum, E.) 27–61 (Springer, New York, 2010).
Joung, Y.-H. Development of implantable medical devices: from an engineering perspective. Int. Neurourol. J. 17, 98–106 (2013).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Leslie, D. C. et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 32, 1134–1140 (2014).
Li, Y. Q., Yu, T., Yang, T. Y., Zheng, L. X. & Liao, K. Bio-Inspired nacre-like composite films based on graphene with superior mechanical, electrical, and biocompatible properties. Adv. Mater. 24, 3426–3431 (2012).
Bixler, G. D. & Bhushan, B. Biofouling: lessons from nature. Philos. Trans. R. Soc. Lond. A 370, 2381–2417 (2012).
Li, L. et al. Multifunctionality of chiton biomineralized armor with an integrated visual system. Science 350, 952–956 (2015).
Liu, K. & Jiang, L. Multifunctional integration: from biological to bio-inspired materials. ACS Nano 5, 6786–6790 (2011).
Huang, Y.-F., Jen, Y.-J., Chen, L.-C., Chen, K.-H. & Chattopadhyay, S. Design for approaching cicada-wing reflectance in low- and high-index biomimetic nanostructures. ACS Nano 9, 301–311 (2015).
Ivanova, E. P. et al. Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 8, 2489–2494 (2012).
Siddique, R. H., Gomard, G. & Hölscher, H. The role of random nanostructures for the omnidirectional anti-reflection properties of the glasswing butterfly. Nat. Commun. 6, 6909 (2015).
Ivanova, E. P. et al. Bactericidal activity of black silicon. Nat. Commun. 4, 2838 (2013).
Kim, S. et al. Nanostructured multifunctional surface with antireflective and antimicrobial characteristics. ACS Appl. Mater. Interf. 7, 326–331 (2015).
Nolte, D. D. Review of centrifugal microfluidic and bio-optical disks. Rev. Sci. Instrum. 80, 101101 (2009).
Lee, J. O. et al. A microscale optical implant for continuous in vivo monitoring of intraocular pressure. Microsyst. 3, 17057 (2017).
Harding, J. L. & Reynolds, M. M. Combating medical device fouling. Trends Biotechnol. 32, 140–146 (2014).
Araci, I. E., Su, B., Quake, S. R. & Mandel, Y. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat. Med. 20, 1074–1078 (2014).
Hasan, J., Crawford, R. J. & Ivanova, E. P. Antibacterial surfaces: the quest for a new generation of biomaterials. Trends Biotechnol. 31, 295–304 (2013).
Quigley, H. A. & Broman, A. T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90, 262–267 (2006).
Peters, D., Bengtsson, B. & Heijl, A. Lifetime risk of blindness in open-angle glaucoma. Am. J. Ophthalmol. 156, 724–730 (2013).
Tham, Y.-C. et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121, 2081–2090 (2014).
Hooper, I. R., Vukusic, P. & Wootton, R. J. Detailed optical study of the transparent wing membranes of the dragonfly Aeshna cyanea. Opt. Express 14, 4891–4897 (2006).
Noh, H. et al. How noniridescent colors are generated by quasi-ordered structures of bird feathers. Adv. Mater. 22, 2871–2880 (2010).
Raut, H. K., Ganesh, V. A., Nair, A. S. & Ramakrishna, S. Anti-reflective coatings: A critical, in-depth review. Energy Environ. Sci. 4, 3779–3804 (2011).
Van de Hulst, H. C. & Twersky, V. Light scattering by small particles. Phys. Today 10, 28–30 (1957).
Khudiyev, T., Huseyinoglu, E. & Bayindir, M. Non-resonant Mie scattering: emergent optical properties of core-shell polymer nanowires. Sci. Rep. 4, 4607 (2014).
Wanasekara, N. D. & Chalivendra, V. B. Role of surface roughness on wettability and coefficient of restitution in butterfly wings. Soft Matter 7, 373–379 (2011).
Huang, C. et al. Polymer blend lithography: a versatile method to fabricate nanopatterned self-assembled monolayers. Beilstein J. Nanotechnol. 3, 620–628 (2012).
Siddique, R. H. et al. Bioinspired phase-separated disordered nanostructures for thin photovoltaic absorbers. Sci. Adv. 3, e1700232 (2017).
Voskerician, G. et al. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials 24, 1959–1967 (2003).
Neumann, A. et al. Comparative investigation of the biocompatibility of various silicon nitride ceramic qualities in vitro. J. Mater. Sci. Mater. Med. 15, 1135–1140 (2004).
Limonov, M. F. & Richard, M. Optical Properties of Photonic Structures: Interplay of Order and Disorder (CRC, Boca Raton, 2012).
Lee, J. H., Lee, S. J., Khang, G. & Lee, H. B. The effect of fluid shear stress on endothelial cell adhesiveness to polymer surfaces with wettability gradient. J. Colloid Interf. Sci. 230, 84–90 (2000).
Lee, J. H. & Lee, H. B. A wettability gradient as a tool to study protein adsorption and cell adhesion on polymer surfaces. J. Biomater. Sci. Polym. Ed. 4, 467–481 (1993).
Banerjee, I., Pangule, R. C. & Kane, R. S. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 23, 690–718 (2011).
Peng, C., Song, S. & Fort, T. Study of hydration layers near a hydrophilic surface in water through AFM imaging. Surf. Interf. Anal. 38, 975–980 (2006).
An, Y. H. & Friedman, R. J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 43, 338–348 (1998).
Wu, P., Hogrebe, P. & Grainger, D. W. DNA and protein microarray printing on silicon nitride waveguide surfaces. Biosens. Bioelectron. 21, 1252–1263 (2006).
Crémet, L. et al. Orthopaedic-implant infections by Escherichia coli: molecular and phenotypic analysis of the causative strains. J. Infect. 64, 169–175 (2012).
Hetrick, E. M. & Schoenfisch, M. H. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 35, 780–789 (2006).
Friedrichs, J., Helenius, J. & Muller, D. J. Quantifying cellular adhesion to extracellular matrix components by single-cell force spectroscopy. Nat. Protoc. 5, 1353–1361 (2010).
Chang, H.-H. et al. Cell adhesion as a novel approach to determining the cellular binding motif on the severe acute respiratory syndrome coronavirus spike protein. J. Virol. Methods 201, 1–6 (2014).
Pogodin, S. et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophysj 104, 835–840 (2013).
Pham, V. T. H. et al. Nanotopography as a trigger for the microscale, autogenous and passive lysis of erythrocytes. J. Mater. Chem. B 2, 2819–2826 (2014).
Weinreb, R. N., Aung, T. & Medeiros, F. A. The pathophysiology and treatment of glaucoma: a review. Jama 311, 1901–1911 (2014).
Dominguez, R. & Holmes, K. C. Actin structure and function. Annu. Rev. Biophys. 40, 169–186 (2011).
Parks, W. C., Wilson, C. L. & Lopez-Boado, Y. S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 4, 617–629 (2004).
Fink, W. et al. Optically powered and optically data-transmitting wireless intraocular pressure sensor device. US patent US20040116794A1 (2004).
Acknowledgements
The work was funded by the National Institute of Health (NIH) research grant EY024582 to H.C. and D.S., a HMRI Investigator Award, Caltech CI2 programme, Powell Foundation Award to H.C., and a Research To Prevent Blindness Innovation Award to D.S. Imaging was performed in the Biological Imaging Facility, with the support of the Caltech Beckman Institute and the Arnold and Mabel Beckman Foundation. We acknowledge support from the Beckman Institute of the California Institute of Technology to the Molecular Materials Research Center.
Author information
Authors and Affiliations
Contributions
V.N., R.H.S. and H.C. conceived the study. V.N. and R.H.S. designed the analyses while supervised by H.C. R.H.S. conducted the microscopy and spectroscopy of the longtail glasswing butterfly. R.H.S. conducted the simulations and numerical analysis. V.N. and R.H.S. fabricated and characterized the nanostructured Si3N4-membrane samples. V.N., R.H.S., S.K. and N.H. conducted the in vitro tests. V.N., J.L. and R.H.S. fabricated and characterized the benchtop IOP sensors. V.N., J.L. and J.D. performed the in vivo experiments under the supervision of D.S., V.N. and B.N. conducted the biocompatibility experiments of the in vivo IOP sensors. V.N., R.H.S. and H.C. co-wrote the manuscript with assistance from D.S. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–3, Supplementary Table 1, Supplementary Figs. 1–20
Rights and permissions
About this article
Cite this article
Narasimhan, V., Siddique, R.H., Lee, J.O. et al. Multifunctional biophotonic nanostructures inspired by the longtail glasswing butterfly for medical devices. Nature Nanotech 13, 512–519 (2018). https://doi.org/10.1038/s41565-018-0111-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0111-5
This article is cited by
-
Aligned CuO nanowire array for a high performance visible light photodetector
Scientific Reports (2022)
-
Recent developments of emerging inorganic, metal and carbon-based nanomaterials for pressure sensors and their healthcare monitoring applications
Nano Research (2021)
-
Engineered disorder in photonics
Nature Reviews Materials (2020)
-
Tunable plasmonic resonances in Si-Au slanted columnar heterostructure thin films
Scientific Reports (2019)
-
Nature-inspired sensors
Nature Nanotechnology (2018)