Abstract
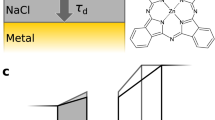
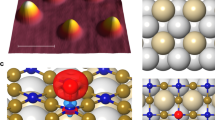
Intermolecular single-electron transfer on electrically insulating films is a key process in molecular electronics1,2,3,4 and an important example of a redox reaction5,6. Electron-transfer rates in molecular systems depend on a few fundamental parameters, such as interadsorbate distance, temperature and, in particular, the Marcus reorganization energy7. This crucial parameter is the energy gain that results from the distortion of the equilibrium nuclear geometry in the molecule and its environment on charging8,9. The substrate, especially ionic films10, can have an important influence on the reorganization energy11,12. Reorganization energies are measured in electrochemistry13 as well as with optical14,15 and photoemission spectroscopies16,17, but not at the single-molecule limit and nor on insulating surfaces. Atomic force microscopy (AFM), with single-charge sensitivity18,19,20,21,22, atomic-scale spatial resolution20 and operable on insulating films, overcomes these challenges. Here, we investigate redox reactions of single naphthalocyanine (NPc) molecules on multilayered NaCl films. Employing the atomic force microscope as an ultralow current meter allows us to measure the differential conductance related to transitions between two charge states in both directions. Thereby, the reorganization energy of NPc on NaCl is determined as (0.8 ± 0.2) eV, and density functional theory (DFT) calculations provide the atomistic picture of the nuclear relaxations on charging. Our approach presents a route to perform tunnelling spectroscopy of single adsorbates on insulating substrates and provides insight into single-electron intermolecular transport.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
19 April 2018
In the version of this Letter originally published, a technical error led to the following spurious text being included "Whis it it that this E_reorg term is differently highlighted than the E_reorg term in the first line of this paragraph? They are the same term."; this text has now been removed from all versions of the Letter.
References
Joachim, C., Gimzewski, J. K. & Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 408, 541–548 (2000).
Ratner, M. A brief history of molecular electronics. Nat. Nanotech. 8, 378–381 (2013).
Tao, N. J. Electron transport in molecular junctions. Nat. Nanotech. 1, 173–181 (2006).
Haiss, W. et al. Precision control of single-molecule electrical junctions. Nat. Mater. 5, 995–1002 (2006).
Moser, C. C., Keske, J. M., Warncke, K., Farid, R. S. & Dutton, P. L. Nature of biological electron transfer. Nature 355, 796–802 (1992).
Adams, D. M. et al. Charge transfer on the nanoscale: current status. J. Phys. Chem. B 107, 6668–6697 (2003).
Marcus, R. A. Electron transfer reactions in chemistry. Theory and Experiment. Rev. Mod. Phys. 65, 599–610 (1993).
Vaissier, V., Barnes, P., Kirkpatrick, J. & Nelson, J. Influence of polar medium on the reorganization energy of charge transfer between dyes in a dye sensitized film. Phys. Chem. Chem. Phys. 15, 4804–4814 (2013).
Brunschwig, B. S., Ehrenson, S. & Sutin, N. Solvent reorganization in optical and thermal electron-transfer processes. J. Phys. Chem. 90, 3657–3668 (1986).
Repp, J., Meyer, G., Paavilainen, S., Olsson, F. E. & Persson, M. Scanning tunneling spectroscopy of Cl vacancies in NaCl films: strong electron–phonon coupling in double-barrier tunneling junctions. Phys. Rev. Lett. 95, 225503 (2005).
Manke, F., Frost, J. M., Vaissier, V., Nelson, J. & Barnes, P. R. F. Influence of a nearby substrate on the reorganization energy of hole exchange between dye molecules. Phys. Chem. Chem. Phys. 17, 7345–7354 (2015).
Moth-Poulsen, K. & Bjørnholm, T. Molecular electronics with single molecules in solid-state devices. Nat. Nanotech. 4, 551–556 (2009).
Eckermann, A. L., Feld, D. J., Shaw, J. A. & Meade, T. J. Electrochemistry of redox-active self-assembled monolayers. Coord. Chem. Rev. 254, 1769–1802 (2010).
Blackbourn, R. L. & Hupp, J. T. Probing the molecular basis of solvent reorganization in electron-transfer reactions. J. Phys. Chem. 92, 2817–2820 (1988).
Bredas, J. L. & Street, G. B. Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 18, 309–315 (1985).
Gruhn, N. E. et al. The vibrational reorganization energy in pentacene: molecular influences on charge transport. J. Am. Chem. Soc. 124, 7918–7919 (2002).
Duhm, S. et al. Charge reorganization energy and small polaron binding energy of rubrene thin films by ultraviolet photoelectron spectroscopy. Adv. Mater. 24, 901–905 (2012).
Stomp, R. et al. Detection of single-electron charging in an individual InAs quantum dot by noncontact atomic-force microscopy. Phys. Rev. Lett. 94, 056802 (2005).
Bussmann, E. & Williams, C. C. Single-electron tunneling force spectroscopy of an individual electronic state in a nonconducting surface. Appl. Phys. Lett. 88, 263108 (2006).
Gross, L. et al. Measuring the charge state of an adatom with noncontact atomic force microscopy. Science 324, 1428–1431 (2009).
Roy-Gobeil, A., Miyahara, Y. & Grutter, P. Revealing energy level structure of individual quantum dots by tunneling rate measured by single-electron sensitive electrostatic force spectroscopy. Nano Lett. 15, 2324–2328 (2015).
Miyahara, Y., Roy-Gobeil, A. & Grutter, P. Quantum state readout of individual quantum dots by electrostatic force detection. Nanotechnology 28, 064001 (2017).
Bevan, K. H. Electron transfer from the perspective of electron transmission: biased non-adiabatic intermolecular reactions in the single-particle picture. J. Chem. Phys. 146, 134106 (2017).
Jortner, J. Temperature dependent activation energy for electron transfer between biological molecules. J. Chem. Phys. 64, 4860–4867 (1976).
Repp, J., Meyer, G., Stojković, S. M., Gourdon, A. & Joachim, C. Molecules on insulating films: scanning-tunneling microscopy imaging of individual molecular orbitals. Phys. Rev. Lett. 94, 026803 (2005).
Feenstra, R. M. Electrostatic potential for a hyperbolic probe tip near a semiconductor. J. Vac. Sci. Technol. B 21, 2080 (2003).
Kera, S. & Ueno, N. Photoelectron spectroscopy on the charge reorganization energy and small polaron binding energy of molecular film. J. Electron. Spectrosc. Relat. Phenom. 204, 2–11 (2015).
Liljeroth, P., Repp, J. & Meyer, G. Current-induced hydrogen tautomerization and conductance switching of naphthalocyanine molecules. Science 317, 1203–1206 (2007).
Imada, H. et al. Real-space investigation of energy transfer in heterogeneous molecular dimers. Nature 538, 364–367 (2016).
Giessibl, F. J. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 73, 3956 (1998).
Albrecht, T. R., Grutter, P., Horne, D. & Rugar, D. Frequency modulation detection using high-Q cantilevers for enhanced force microscope sensitivity. J. Appl. Phys. 69, 668 (1991).
Steurer, W., Gross, L. & Meyer, G. Local thickness determination of thin insulator films via localized states. Appl. Phys. Lett. 104, 231606 (2014).
Steurer, W., Fatayer, S., Gross, L. & Meyer, G. Probe-based measurement of lateral single-electron transfer between individual molecules. Nat. Commun. 6, 8353 (2015).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Scivetti, I. & Persson, M. The electrostatic interaction of an external charged system with a metal surface: a simplified density functional theory approach. J. Phys. Condens. Matter 25, 355006 (2013).
Scivetti, I. & Persson, M. A simplified density functional theory method for investigating charged adsorbates on an ultrathin, insulating film supported by a metal substrate. J. Phys. Condens. Matter 26, 135003 (2014).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Klimeš, J., Bowler, D. R. & Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 83, 195131 (2011).
Dion, M., Rydberg, H., Schröder, E., Langreth, D. C. & Lundqvist, B. I. Van der Waals density functional for general geometries. Phys. Rev. Lett. 92, 246401 (2004).
Thonhauser, T. et al. Van der Waals density functional: self-consistent potential and the nature of the van der Waals bond. Phys. Rev. B 76, 125112 (2007).
Román-Pérez, G. & Soler, J. M. Efficient implementation of a van der Waals density functional: application to double-wall carbon nanotubes. Phys. Rev. Lett. 103, 096102 (2009).
Repp, J. et al. Charge-state-dependent diffusion of individual gold adatoms on ionic thin NaCl films. Phys. Rev. Lett. 117, 146102 (2016).
Scivetti, I. & Persson, M. Frontier molecular orbitals of a single molecule adsorbed on thin insulating films supported by a metal substrate: electron and hole attachment energies. J. Phys. Condens. Matter 29, 355002 (2017).
Björk, J. et al. Adsorption of aromatic and anti-aromatic systems on graphene through π–π stacking. J. Phys. Chem. Lett. 1, 3407–3412 (2010).
Acknowledgements
We thank R. Allenspach for comments. Financial support by the European Research Council (advanced grant ‘CEMAS’, agreement no. 291194, and consolidator grant ‘AMSEL’, agreement no. 682144), EU projects ‘PAMS’ (contract no. 610446) and Initial Training Network ‘ACRITAS’ (contract no. 317348). The Leverhulme Trust (F/00 025/AQ) and the allocations of computer resources at Chadwick, The University of Liverpool, are gratefully acknowledged. I.S. acknowledges CCP5 funding and associated CoSeC support at STFC via EPSRC grant no. EP/M022617/1 and SLA for funding.
Author information
Authors and Affiliations
Contributions
S.F, W.S., J.R., L.G. and G.M. designed the experiments. S.F., B.S., L.G. and G.M. performed the experiments. S.F. carried out the finite-element simulations. M.P. and I.S. were responsible for the DFT calculations. All the authors discussed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–9, Supplementary Table 1
Rights and permissions
About this article
Cite this article
Fatayer, S., Schuler, B., Steurer, W. et al. Reorganization energy upon charging a single molecule on an insulator measured by atomic force microscopy. Nature Nanotech 13, 376–380 (2018). https://doi.org/10.1038/s41565-018-0087-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0087-1
This article is cited by
-
Charge-state lifetimes of single molecules on few monolayers of NaCl
Nature Communications (2023)
-
Voltage-driven control of single-molecule keto-enol equilibrium in a two-terminal junction system
Nature Communications (2023)
-
A DFT Study on the Relationship Between Molecular Structure and Electron Transport in Molecular Junctions
Journal of Electronic Materials (2023)
-
Orbital-resolved visualization of single-molecule photocurrent channels
Nature (2022)
-
Internal Stark effect of single-molecule fluorescence
Nature Communications (2022)