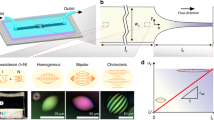
Abstract
Chirality is ubiquitous in nature and plays crucial roles in biology, medicine, physics and materials science. Understanding and controlling chirality is therefore an important research challenge with broad implications. Unlike other chiral colloids, such as nanocellulose or filamentous viruses, amyloid fibrils form nematic phases but appear to miss their twisted form, the cholesteric or chiral nematic phases, despite a well-defined chirality at the single fibril level. Here we report the discovery of cholesteric phases in amyloids, using β-lactoglobulin fibrils shortened by shear stresses. The physical behaviour of these new cholesteric materials exhibits unprecedented structural complexity, with confinement-driven ordering transitions between at least three types of nematic and cholesteric tactoids. We use energy functional theory to rationalize these results and observe a chirality inversion from the left-handed amyloids to right-handed cholesteric droplets. These findings deepen our understanding of cholesteric phases, advancing their use in soft nanotechnology, nanomaterial templating and self-assembly.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Salam, A. The role of chirality in the origin of life. J. Mol. Evol. 33, 105–113 (1991).
Ramström, O. & Ansell, R. J. Molecular imprinting technology: challenges and prospects for the future. Chirality 10, 195–209 (1998).
Bradshaw, D., Claridge, J. B., Cussen, E. J., Prior, T. J. & Rosseinsky, M. J. Design, chirality, and flexibility in nanoporous molecule-based materials. Acc. Chem. Res. 38, 273–282 (2005).
Shopsowitz, K. E., Qi, H., Hamad, W. Y. & Maclachlan, M. J. Free-standing mesoporous silica films with tunable chiral nematic structures. Nature 468, 422–425 (2010).
Gibaud, T. et al. Reconfigurable self-assembly through chiral control of interfacial tension. Nature 481, 348–351 (2012).
Dobson, C. M. Protein folding and misfolding. Nature 426, 884–890 (2002).
Glenner, G. G. Amyloid deposits and amyloidosis: (first of two parts). N. Engl. J. Med. 302, 1283–1292 (1980).
Maddelein, M.-L., Dos Reis, S., Duvezin-Caubet, S., Coulary-Salin, B. & Saupe, S. J. Amyloid aggregates of the HET-s prion protein are infectious. Proc. Natl Acad. Sci. USA 99, 7402–7407 (2002).
Barnhart, M. M. & Chapman, M. R. Curli biogenesis and function. Annu. Rev. Microbiol. 60, 131–147 (2006).
Maji, S. K. et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science 325, 328–332 (2009).
Adamcik, J. et al Understanding amyloid aggregation by statistical analysis of atomic force microscopy images. Nat. Nanotech. 5, 423–428 (2010).
Knowles, T. P. J. & Mezzenga, R. Amyloid fibrils as building blocks for natural and artificial functional materials. Adv. Mater. 28, 6546–6561 (2016).
Usov, I., Adamcik, J. & Mezzenga, R. Polymorphism complexity and handedness inversion in serum albumin amyloid fibrils. ACS Nano 7, 10465–10474 (2013).
Kitzerow, H. & Bahr, C. Chirality in Liquid Crystals. (Springer: New York, 2001.
Robinson, C. Liquid-crystalline structures in polypeptide solutions. Tetrahedron 13, 219–234 (1961).
Dogic, Z. & Fraden, S. Cholesteric phase in virus suspensions. Langmuir 16, 7820–7824 (2000).
Mosser, G., Anglo, A., Helary, C., Bouligand, Y. & Giraud-Guille, M. M. Dense tissue-like collagen matrices formed in cell-free conditions. Matrix Biol. 25, 3–13 (2006).
Revol, J., Bradford, H., Giasson, J., Marchessault, R. H. & Gray, D. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 14, 170–172 (1992).
Bonazzi, S. et al. Four-stranded aggregates of oligodeoxyguanylates forming lyotropic liquid crystals: a study by circular dichroism, optical microscopy, and x-ray diffraction. J. Am. Chem. Soc. 113, 5809–5816 (1991).
Livolant, F. & Maestre, M. F. Circular dichroism microscopy of compact forms of DNA and chromatin in vivo and in vitro: cholesteric liquid-crystalline phases of DNA and single dinoflagellate nuclei. Biochemistry 27, 3056–3068 (1988).
Tombolato, F., Ferrarini, A. & Grelet, E. Chiral nematic phase of suspensions of rodlike viruses: left-handed phase helicity from a right-handed molecular helix. Phys. Rev. Lett. 96, 258302 (2006).
Usov, I. & Mezzenga, R. FiberApp: An open-source software for tracking and analyzing polymers, filaments, biomacromolecules, and fibrous objects. Macromolecules 48, 1269–1280 (2015).
Lara, C., Adamcik, J., Jordens, S. & Mezzenga, R. General self-assembly mechanism converting hydrolyzed globular proteins into giant multistranded amyloid ribbons. Biomacromolecules 12, 1868–1875 (2011).
Mezzenga, R., Jung, J.-M. & Adamcik, J. Effects of charge double layer and colloidal aggregation on the isotropic-nematic transition of protein fibers in water. Langmuir 26, 10401–10405 (2010).
Vroege, G. J. & Lekkerkerker, H. N. W. Phase transitions in lyotropic colloidal and polymer liquid crystals. Rep. Prog. Phys. 55, 1241–1309 (1992).
Khokhlov, A. & Semenov, A. Liquid-crystalline ordering in the solution of long persistent chains. Phys. A 108, 546–556 (1981).
Bouligand, Y. & Livolant, F. The organization of cholesteric spherulites. J. Phys. 45, 1899–1923 (1984).
Prinsen, P. & van der Schoot, P. Shape and director-field transformation of tactoids. Phys. Rev. E 68, 21701 (2003).
Jamali, V. et al. Experimental realization of crossover in shape and director field of nematic tactoids. Phys. Rev. E 91, 42507 (2015).
Tortora, L. & Lavrentovich, O. D. Chiral symmetry breaking by spatial confinement in tactoidal droplets of lyotropic chromonic liquid crystals. Proc. Natl Acad. Sci. USA 108, 5163–5168 (2011).
Nayani, K. et al. Spontaneous emergence of chirality in achiral lyotropic chromonic liquid crystals confined to cylinders. Nat. Commun. 6, 8067 (2015).
van der Schoot, P. Remarks on the interfacial tension in colloidal systems. J. Phys. Chem. B 103, 8804–8808 (1999).
Straley, J. P. Theory of piezoelectricity in nematic liquid crystals, and of the cholesteric ordering. Phys. Rev. A 14, 1835–1841 (1976).
Frezza, E., Ferrarini, A., Kolli, H. B., Giacometti, A. & Cinacchi, G. Left or right cholesterics? A matter of helix handedness and curliness. Phys. Chem. Chem. Phys. 16, 16225–16232 (2014).
Wensink, H. H. & Ferreiro-Córdova, C. Twisting with a twist: supramolecular helix fluctuations in chiral nematics. Soft Matter 13, 3885–3893 (2017).
Zanchetta, G. et al. Right-handed double-helix ultrashort DNA yields chiral nematic phases with both right- and left-handed director twist. Proc. Natl Acad. Sci. USA 107, 17497–17502 (2010).
Frezza, E., Tombolato, F. & Ferrarini, A. Right- and left-handed liquid crystal assemblies of oligonucleotides: phase chirality as a reporter of a change in non-chiral interactions? Soft Matter 7, 9291 (2011).
Belli, S., Dussi, S., Dijkstra, M. & van Roij, R. Density functional theory for chiral nematic liquid crystals. Phys. Rev. E 90, 20503 (2014).
Acknowledgements
We acknowledge support from the Scientific Center for Optical and Electron Microscopy of ETH Zurich (ScopeM). S. Handschin and T. Schwarz are acknowledged for help with the sample rotation stage for polarized optical microscopy and the laser scanning confocal microscopy, respectively. S. Assenza and C. de Michele are acknowledged for discussions.
Author information
Authors and Affiliations
Contributions
G.N. designed and carried out the experiments, analysed data and interpreted the results. M.A. carried out the experiments, analysed data and interpreted the results. R.M. developed the theoretical description, analysed and interpreted the results, and designed and directed the study. All authors discussed the results and contributed to writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussion, Supplementary Figures 1–5, captions for Supplementary Videos 1–4.
Supplementary Video 1
Rotation of a cholesteric tactoid around an axis parallel to the observation plane and passing through the poles of the apparent spindle-like droplet.
Supplementary Video 2
Coalescence of two nematic tactoids and their transition into one cholesteric tactoid.
Supplementary Video 3
Right-handed rotation of a cholesteric tactoid.
Supplementary Video 4
Right-handed rotation of a right-handed rod helix.
Rights and permissions
About this article
Cite this article
Nyström, G., Arcari, M. & Mezzenga, R. Confinement-induced liquid crystalline transitions in amyloid fibril cholesteric tactoids. Nature Nanotech 13, 330–336 (2018). https://doi.org/10.1038/s41565-018-0071-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0071-9
This article is cited by
-
Self-assembling synthetic polymer forms liquid-like droplets
Nature (2024)
-
Supramolecular polymers form tactoids through liquid–liquid phase separation
Nature (2024)
-
Fluid protein condensates for bio-inspired applications
Nature Reviews Bioengineering (2023)
-
Disentangling kinetics from thermodynamics in heterogeneous colloidal systems
Nature Communications (2023)
-
Hierarchical metal-peptide assemblies with chirality-encoded spiral architecture and catalytic activity
Science China Chemistry (2023)