Abstract
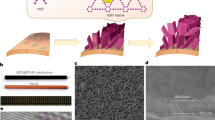
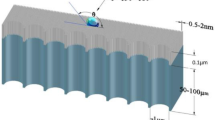
Freshwater flux and energy consumption are two important benchmarks for the membrane desalination process. Here, we show that nanoporous carbon composite membranes, which comprise a layer of porous carbon fibre structures grown on a porous ceramic substrate, can exhibit 100% desalination and a freshwater flux that is 3–20 times higher than existing polymeric membranes. Thermal accounting experiments demonstrated that the carbon composite membrane saved over 80% of the latent heat consumption. Theoretical calculations combined with molecular dynamics simulations revealed the unique microscopic process occurring in the membrane. When the salt solution is stopped at the openings to the nanoscale porous channels and forms a meniscus, the vapour can rapidly transport across the nanoscale gap to condense on the permeate side. This process is driven by the chemical potential gradient and aided by the unique smoothness of the carbon surface. The high thermal conductivity of the carbon composite membrane ensures that most of the latent heat is recovered.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Elimelech, M. & Phillip, W. A. The future of seawater desalination: energy, technology and the environment. Science 333, 712–717 (2011).
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–309 (2008).
Cipollina, A. et al. (eds) Seawater Desalination (Springer, Heidelberg, 2009).
Ghaffour, N., Missimer, T. M. & Amy, G. L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 309, 197–207 (2013).
El-Ghonemy, A. M. K. Water desalination systems powered by renewable energy sources: Review. Renew. Sust. Energy Rev. 16, 1537–1556 (2012).
Kumar, M., Habel, J. E. O., Shen, Y. X., Meier, W. P. & Walz, T. High-density reconstitution of functional water channels into vesicular and planar block copolymer membranes. J. Am. Chem. Soc. 134, 18631–18637 (2012).
Holt, J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312, 1034–1037 (2006).
Theresa, M., Pendergast, M. & Hoek, M. V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 4, 1946–1971 (2011).
Verweij, H., Schillo, M. C. & Li, J. Fast mass transport through carbon nanotube membranes. Small 3, 1996–2004 (2007).
Song, C. & Corry, B. Intrinsic ion selectivity of narrow hydrophobic pores. J. Phys. Chem. B 113, 7642–7649 (2009).
Corry, B. Water and ion transport through functionalised carbon nanotubes: implications for desalination technology. Energy Environ. Sci. 4, 751–759 (2011).
Srivastava, A., Srivastava, O. N., Talapatra, S., Vajtai, R. & Ajayan, P. M. Carbon nanotube filters. Nat. Mater. 3, 610–614 (2004).
Tofighy, M. A., Shirazi, Y., Mohammadi, R. & Park, A. Salty water desalination using carbon nanotubes membrane. Chem. Eng. J. 168, 1064–1072 (2011).
Majumder, M. & Ajayan, P. M. in Comprehensive Membrane Science and Engineering (eds E. Drioli & L. Giorno) 291–310 (Elsevier, UK, 2010).
Mi, W. L., Lin, Y. S. & Li, Y. D. Vertically aligned carbon nanotube membranes on macroporous alumina supports. J. Membr. Sci. 304, 1–7 (2007).
Kim, S., Jinschek, J. R., Chen, H., Sholl, D. S. & Marand, E. Scalable fabrication of carbon nanotube/polymer nanocomposite membranes for high flux gas transport. Nano Lett. 7, 2806–2811 (2007).
Zhong, Y. J. et al. Using UCST ionic liquid as a draw solute in forward osmosis to treat high-salinity water. Environ. Sci. Technol. 50, 1039–1045 (2016).
Ratto, T. V., Holt, J. K. & Szmodis A. W. Membranes with embedded nanotubes for selective permeability. US patent 7,993,524 (2011).
Lee, H. D., Kim, H. W., Cho, Y. H. & Park, H. B. Experimental evidence of rapid water transport through carbon nanotubes embedded in polymeric desalination membranes. Small 10, 2653–2660 (2014).
Nair, R. R., Wu, H. A., Jayaram, P. N., Grigorieva, I. V. & Geim, A. K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 335, 442–444 (2012).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Kim, H. W. et al. Selective gas transport through few-layered graphene and graphene oxide membranes. Science 342, 91–95 (2013).
Abraham, J. et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotech. 12, 546–551 (2017).
Yasuda, H. & Tsai, J. T. Pore size of microporous polymer membranes. J. Appl. Polym. Sci. 18, 805–819 (1974).
Wang, P., Teoh, M. M. & Chung, T. S. Morphological architecture of dual-layer hollow fiber for membrane distillation with higher desalination performance. Water Res. 45, 5489–5500 (2011).
Fornasiero, F. et al. Ion exclusion by sub-2-nm carbon nanotube pores. Proc. Natl Acad. Sci. USA 105, 17250–17255 (2008).
Schaep, J., Vandecasteele, C., Mohammad, A. W. & Bowen, W. R. Modelling the retention of ionic components for different nanofiltration membranes. Sep. Purif. Technol. 22–23, 169–179 (2001).
Peeters, J. M. M., Boom, J. P., Mulder, M. H. V. & Srathmann, H. Retention measurements of nanofiltration membranes with electrolyte solutions. J. Membr. Sci. 145, 199–209 (1998).
Chekli, L. et al. A comprehensive review of hybrid forward osmosis systems: performance, applications and future prospects. J. Membr. Sci. 497, 430–449 (2016).
Geise, G. M., Park, H. B., Sagle, A. C., Freeman, B. D. & Mcgrath, J. E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 369, 130–138 (2011).
Dang, L. X., Rice, J. E., Caldwell, J. & Kollman, P. A. Ion solvation in polarizable water: molecular dynamics simulations. J. Am. Chem. Soc. 113, 2481–2486 (1991).
Mancinelli, R., Botti, A., Bruni, F., Ricci, M. A. & Soper, A. K. Hydration of sodium, potassium, and chloride ions in solution and the concept of structure maker/breaker. J. Phys. Chem. B 111, 13570–13577 (2007).
Taherian, F., Marcon, V., van der Vegt, N. F. A. & Leroy, F. What is the contact angle of water on graphene? Langmuir 29, 1457–1465 (2013).
Kimmel, G. A. et al. No confinement needed: observation of a metastable hydrophobic wetting two-layer ice on graphene. J. Am. Chem. Soc. 131, 12838–12844 (2009).
Lee, J. & Karnik, R. Desalination of water by vapor-phase transport through hydrophobic nanopores. J. Appl. Phys. 108, 044315 (2010).
Skoulidas, A. I., Ackerman, D. M., Johnson, J. K. & Sholl, D. S. Rapid transport of gases in carbon nanotubes. Phys. Rev. Lett. 89, 185901 (2002).
Arya, G., Chang, H.-C. & Maginn, E. Knudsen diffusivity of a hard sphere in a rough slit pore. Phys. Rev. Lett. 91, 026102 (2003).
Falk, K., Sedlmeier, F., Joly, L., Netz, R. R. & Bocquet, L. Molecular origin of fast water transport in carbon nanotube membranes: superlubricity versus curvature dependent friction. Nano Lett. 10, 4067–4073 (2010).
Liu, S. M., Li, K. & Hughes, R. Preparation of porous aluminium oxide (Al2O3) hollow fibre membranes by a combined phase-inversion and sintering method. Ceram. Int. 29, 875–881 (2003).
Wang, B. & Lai, Z. P. Finger-like voids induced by viscous fingering during phase-inversion of alumina/PES/NMP suspensions. J. Membr. Sci. 405–406, 275–283 (2012).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comp. Chem. 26, 1701–1718 (2005).
Weerasinghe, S. & Smith, P. E. A Kirkwood–Buff derived force field for sodium chloride in water. J. Chem. Phys. 119, 11342–11349 (2003).
Werder, T., Walther, J. H., Jaffe, R., Halicioglu, T. & Koumoutsakos, P. On the water–carbon interaction for use in molecular dynamics simulations of graphite and carbon nanotubes. J. Phys. Chem. B 107, 1345–1352 (2003).
Oostenbrink, C., Villa, A., Mark, A. E. & van Gunsteren, W. F. A biomolecular force field based on the free enthalpy of hydration and solvation: the GROMOS force‐field parameter sets 53A5 and 53A6. J. Comp. Chem. 25, 1656–1676 (2004).
Ryckaert, J. P., Ciccotti, G. & Berendsen, H. J. Numerical integration of the Cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comp. Phys. 23, 327–341 (1977).
Berendsen, H. J., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Vanegas, J. M., Torres-Sánchez, A. & Arroyo, M. Importance of force decomposition for local stress calculations in biomembrane molecularsimulations. J. Chem. Theory Comput. 10, 691–702 (2014).
Acknowledgements
Commercial PTFE membranes and FO membranes were provided by N. Ghaffour and T. Zhang from the KAUST Water Desalination and Reuse Center. Z.L. acknowledges support from KAUST (grant URF/1/1723) and KACST (grant RGC/3/1614). P.S. acknowledges support from KAUST (Special Partnerships Award number UK-C0016 and grant SA-C0040), HKUST (grant SRFI 11/SC02) and the William Mong Institute of Nanoscience and Technology (grant G5537-E).
Author information
Authors and Affiliations
Contributions
Z.L. and W.C. conceived the initial ideas and experimental design. S.C., T.L. and P.S. contributed to the desalination mechanism and the relevant simulations and data analyses. W.C., Q.Z., Z.F. and H.Y. carried out the experiments, and T.L. and S.C. carried out the MD simulations. K.-W.H. contributed to data analyses, and X.Z. contributed to characterization and data analyses. Z.L., W.C. and P.S. wrote the first draft, and Z.L., W.C., P.S., S.C., T.L. and X.Z. participated in the revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisherʼs note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Text, Supplementary Figures 1–15 and Supplementary Tables 1–9.
Rights and permissions
About this article
Cite this article
Chen, W., Chen, S., Liang, T. et al. High-flux water desalination with interfacial salt sieving effect in nanoporous carbon composite membranes. Nature Nanotech 13, 345–350 (2018). https://doi.org/10.1038/s41565-018-0067-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0067-5
This article is cited by
-
Graphdiyne membranes for ultrafast desalination
Nature Water (2023)
-
Ultrahigh-water-flux desalination on graphdiyne membranes
Nature Water (2023)
-
A super liquid-repellent hierarchical porous membrane for enhanced membrane distillation
Nature Communications (2023)
-
A review of membrane distillation enhancement via thermal management and molecular transport through nanomaterial-based membranes
Science China Technological Sciences (2023)
-
Synergistic realization of high efficiency solar desalination and carbon dioxide reduction
Nano Research (2023)