Abstract
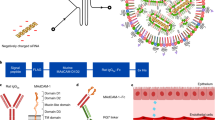
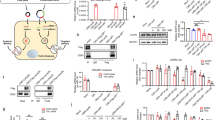
Previous studies have identified relevant genes and signalling pathways that are hampered in human disorders as potential candidates for therapeutics. Developing nucleic acid-based tools to manipulate gene expression, such as short interfering RNAs1,2,3 (siRNAs), opens up opportunities for personalized medicine. Yet, although major progress has been made in developing siRNA targeted delivery carriers, mainly by utilizing monoclonal antibodies (mAbs) for targeting4,5,6,7,8, their clinical translation has not occurred. This is in part because of the massive development and production requirements and the high batch-to-batch variability of current technologies, which rely on chemical conjugation. Here we present a self-assembled modular platform that enables the construction of a theoretically unlimited repertoire of siRNA targeted carriers. The self-assembly of the platform is based on a membrane-anchored lipoprotein that is incorporated into siRNA-loaded lipid nanoparticles that interact with the antibody crystallizable fragment (Fc) domain. We show that a simple switch of eight different mAbs redirects the specific uptake of siRNAs by diverse leukocyte subsets in vivo. The therapeutic potential of the platform is demonstrated in an inflammatory bowel disease model by targeting colon macrophages to reduce inflammatory symptoms, and in a Mantle Cell Lymphoma xenograft model by targeting cancer cells to induce cell death and improve survival. This modular delivery platform represents a milestone in the development of precision medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dahlman, J. E. et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 9, 648–655 (2014).
McNamara, J. O. II et al. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 24, 1005–1015 (2006).
Wittrup, A. & Lieberman, J. Knocking down disease: a progress report on siRNA therapeutics. Nat. Rev. Genet. 16, 543–552 (2015).
Ramishetti, S. et al. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano 9, 6706–6716 (2015).
Weinstein, S. et al. Harnessing RNAi-based nanomedicines for therapeutic gene silencing in B-cell malignancies. Proc. Natl Acad. Sci. USA 113, E16–E22 (2016).
Katakowski, J. A. et al. Delivery of siRNAs to dendritic cells using DEC205-targeted lipid nanoparticles to inhibit immune responses. Mol. Ther. 24, 146–155 (2016).
Peer, D., Zhu, P., Carman, C. V., Lieberman, J. & Shimaoka, M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc. Natl Acad. Sci. USA 104, 4095–4100 (2007).
Song, E. et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 23, 709–717 (2005).
Springer, T. A., Bhattacharya, A., Cardoza, J. T. & Sanchez-Madrid, F. Monoclonal antibodies specific for rat IgG1, IgG2a, and IgG2b subclasses, and kappa chain monotypic and allotypic determinants: reagents for use with rat monoclonal antibodies. Hybridoma 1, 257–273 (1982).
Laukkanen, M. L., Teeri, T. T. & Keinanen, K. Lipid-tagged antibodies: bacterial expression and characterization of a lipoprotein-single-chain antibody fusion protein. Protein Eng. 6, 449–454 (1993).
de Kruif, J., Storm, G., van Bloois, L. & Logtenberg, T. Biosynthetically lipid-modified human scFv fragments from phage display libraries as targeting molecules for immunoliposomes. FEBS Lett. 399, 232–236 (1996).
Semple, S. C. et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28, 172–176 (2010).
Cohen, Z. R. et al. Localized RNAi therapeutics of chemoresistant grade IV glioma using hyaluronan-grafted lipid-based nanoparticles. ACS Nano. 9, 1581–1591 (2015).
Tiisala, S., Paavonen, T. & Renkonen, R. Alpha E beta 7 and alpha 4 beta 7 integrins associated with intraepithelial and mucosal homing, are expressed on macrophages. Eur. J. Immunol. 25, 411–417 (1995).
Peer, D., Park, E. J., Morishita, Y., Carman, C. V. & Shimaoka, M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319, 627–630 (2008).
Zigmond, E. et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090 (2012).
Grainger, J. R. et al. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat. Med. 19, 713–721 (2013).
Benhar, I. & Pastan, I. Cloning, expression and characterization of the Fv fragments of the anti-carbohydrate mAbs Bl and B5 as single-chain immunotoxins. Protein Eng. 7, 1509–1515 (1994).
Vaks, L. & Benhar, I. Production of stabilized scFv antibody fragments in the E. coli bacterial cytoplasm. Methods Mol. Biol. 1060, 171–184 (2014).
Acknowledgements
This work was supported in part by grants from the Dotan Hemato-oncology Center at Tel Aviv University, by The Leona M. and Harry B. Helmsley Nanotechnology Research Fund, by the Kenneth Rainin Foundation and by the ERC grant LeukoTheranostics (number 647410) awarded to D.P.
Author information
Authors and Affiliations
Contributions
R.K., N.V. and D.P. conceived and designed the project. R.K., N.V. S.R., M.G., D.R. N.D., L.N., I.H.-H., S.L.-B.-A. and M.H. performed the experimental work. R.K., N.V., M.B., I.B. J.L. and D.P. analysed the data. R.K. N.V. and D.P. wrote the manuscript. All authors discussed the results.
Corresponding author
Ethics declarations
Competing interests
M.B. is an employee of Integrated DNA Technologies, Inc. J.L. is on the Scientific Advisory Board of Alnylam Pharmaceuticals. D.P. declares financial interests in Quiet Therapeutics. The rest of the authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figures
Supplementary Figures 1–6.
Rights and permissions
About this article
Cite this article
Kedmi, R., Veiga, N., Ramishetti, S. et al. A modular platform for targeted RNAi therapeutics. Nature Nanotech 13, 214–219 (2018). https://doi.org/10.1038/s41565-017-0043-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-017-0043-5
This article is cited by
-
Strategies for non-viral vectors targeting organs beyond the liver
Nature Nanotechnology (2024)
-
Nanomedicine in cancer therapy
Signal Transduction and Targeted Therapy (2023)
-
Targeting cancer with mRNA–lipid nanoparticles: key considerations and future prospects
Nature Reviews Clinical Oncology (2023)
-
Drug delivery systems for CRISPR-based genome editors
Nature Reviews Drug Discovery (2023)
-
CRISPR editing in the lung with novel lipids
Nature Biotechnology (2023)