Abstract
Vaccines and monoclonal antibody treatments to prevent severe coronavirus disease 2019 (COVID-19) illness were available within a year of the pandemic being declared but there remained an urgent need for therapeutics to treat patients who were not vaccinated, were immunocompromised or whose vaccine immunity had waned. Initial results for investigational therapies were mixed. AT-527, a repurposed nucleoside inhibitor for hepatitis C virus, enabled viral load reduction in a hospitalized cohort but did not reduce viral load in outpatients. The nucleoside inhibitor molnupiravir prevented death but failed to prevent hospitalization. Nirmatrelvir, an inhibitor of the main protease (Mpro), co-dosed with the pharmacokinetic booster ritonavir, reduced hospitalization and death. Nirmatrelvir–ritonavir and molnupiravir received an Emergency Use Authorization in the United States at the end of 2021. Immunomodulatory drugs such as baricitinib, tocilizumab and corticosteroid, which target host-driven COVID-19 symptoms, are also in use. We highlight the development of COVID-19 therapies and the challenges that remain for anticoronavirals.
Similar content being viewed by others
Main
More than three years into the coronavirus disease 2019 (COVID-19) pandemic, >757 million confirmed cases, including >6.8 million deaths, have been reported worldwide as of 21 February 2023 (ref. 1). COVID-19 is the third coronavirus disease in the past 20 years2. Although severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) were severe in terms of mortality rates, they failed to spread around the world3.
COVID-19 disease is highly variable. An exploratory analysis of 72,314 cases in China in February 2020 reported that most (81%) infected individuals develop mild-to-moderate disease, 14% develop severe respiratory disease and 5% progress to critical illness including respiratory failure4,5. Mild disease includes fever, respiratory symptoms (cough and sore throat), a loss of taste and/or smell, headache, myalgias and gastrointestinal symptoms (nausea, vomiting and/or diarrhoea). Individuals with moderate disease have lower respiratory tract illness with symptoms that can include shortness of breath with exertion and signs of moderate pneumonia, for example, a respiratory rate of 20–29 breaths per minute, yet able to maintain 94% oxygen saturation on room air at sea level. Severe COVID-19 can manifest with shortness of breath at rest, respiratory distress, including progression of clinical signs to a respiratory rate of ≥30 breaths per minute, an oxygen saturation of ≤ 93% on room air at sea level or a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen of <300 mm Hg. Critical disease is defined by evidence of respiratory failure, shock or multi-organ failure. Those with severe and critical disease are at increased risk of death or developing complications including arrhythmias, acute kidney injury, thrombo-embolic events and septic shock6,7. Even in those that recover from COVID-19 infection, irrespective of the acuity of disease, some people have persistent symptoms, known as long COVID8,9.
The US Centers for Disease Control and Prevention (CDC) COVID-19 case surveillance report analysed 1.3 million COVID-19 cases in the United States between January and May 2020, and found that the hospitalization rate was 14%, 2% required intensive care and, overall, 5.4% died10. Populations at increased risk of severe disease and hospitalization include those older than 60 yr or with specific co-morbidities including hypertension, diabetes, cardiovascular disease, chronic lung disease and obesity10,11,12. Risk factors for death due to COVID-19 include male sex, Black race, older age and underlying medical conditions, including chronic kidney disease and cardiovascular disease13. Institutionalized individuals, including older individuals living in residential facilities or incarcerated persons, also have an increased mortality risk14,15. In a CDC report characterizing COVID-19 among assisted-living facilities with available data across the United States, COVID-19 infections were fatal in 21.4% of 24,435 residents, compared with a 2.5% overall fatality in the general population (4.7 million) of those states16.
A global vaccination campaign sought to prevent disease, disrupt transmission of the virus and reduce the need for therapeutics. Several vaccines have been approved by the World Health Organization (WHO) for the prevention of COVID-19 (ref. 17). However, there are many obstacles to global vaccination: time, resources to manufacture vaccines, costs and availability of personnel to immunize people, varying levels of vaccine efficacy and durability, limited response to vaccination in immunocompromised patients, vaccine hesitancy, emergence of more transmissible variants and finally, future pandemic coronaviruses that we have not seen yet. Therefore, drugs to treat SARS coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, are still needed.
Monoclonal antibody therapies for COVID-19 were available 10 months after the pandemic was declared18,19,20 but variants have rendered many of these ineffective21,22. Oral therapies that can be administered outside of hospitals are a vital tool in fighting the pandemic. So far, two oral therapeutics have been authorized in The US and Europe: nirmatrelvir administered with ritonavir (Pfizer)23 and molnupiravir (Merck)24.
Here we review the approaches taken by pharmaceutical companies, government agencies and academia working together to develop COVID-19 therapeutics.
Identifying therapeutic targets
Antiviral design requires an understanding of the virus and the clinical outcomes of infection. For SARS-CoV-2, infection can be divided into four stages that are characterized by distinct clinical signs and symptoms increasing in severity as the disease progresses that require different interventions (Fig. 1a)5,25,26,27,28. Stage 0, preceding SARS-CoV-2 infection and/or exposure, is the ideal point for prophylactic administration of vaccines, neutralizing antibodies or antivirals with an acceptable safety profile. Antibody and antiviral therapies, administered intravenously or orally during Stage 1, will also have an impact because this is when viral replication occurs. At the beginning of the pandemic there were uncertainties around the length of this stage. SARS-CoV-2 initially causes an acute viral infection akin to influenza. Antiviral drug treatment for influenza needs to be administered within the first 48 h of symptom onset29. Through clinical trials, we know that antibodies for COVID-19 treatment can be administered up to 10 d after symptom onset30,31 and oral antivirals can be administered within 3–5 d following the onset of symptoms to be effective32,33,34,35,36. Studies have shown that SARS-CoV-2 viral replication peaks by approximately 3 d into symptom onset and tends to clear in 5.9–7.6 d, depending on vaccination status, after which the clinical manifestations and disease severity are driven by a dysregulated immune response to SARS-CoV-2, as shown in Stages 2 and 3 (refs. 37,38,39). As diagnostics have become more available, it is clear that for some patients the virus can linger and that later detection of the virus can be associated with symptom relapse40.
The SARS-CoV-2 lifecycle and potential targets for antiviral therapies are shown in Fig. 1b. In Stage 2 patients develop a viral pneumonia with cough and fever, and prominent lung inflammation causes hypoxia and shortness of breath, which may or may not require oxygen supplementation. Chest imaging reveals characteristic lung abnormalities such as bilateral infiltrates or ground glass opacities28. Stage 3, the most severe disease, is a hyperinflammatory state characterized by acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome (SIRS)/shock, coagulation disorders and cardiac failure, with a very high mortality rate. Patients in Stages 2 and 3 need agents that target the host inflammatory response in addition to antivirals, and immunomodulatory therapy is often required in Stage 3 (Fig. 1a). The cascade of inflammatory reactions seen in patients with advanced COVID-19, which might be affected by immunomodulatory agents, is shown in Fig. 1c. Patients who progress to Stage 3 also often require mechanical ventilation (Fig. 1a)28. Some individuals may experience post-COVID conditions (Stage 4) following acute infection, which can include post-acute hyperinflammatory illnesses such as multi-system inflammatory syndrome in children and adults or late sequelae such as long COVID41,42.
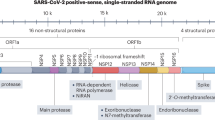
a, Disease time course, symptoms and interventions. SIRS, systemic inflammatory response syndrome; TBD, to be determined. b, The SARS-CoV-2 lifecycle. SARS-CoV-2 enters host cells via binding of the viral spike protein to the ACE2 receptor and the TMPRSS2-mediated viral membrane fusion with the plasma membrane, followed by pre-activation of the spike protein by proprotein convertase furin and further processing by cathepsin in the endosome as well as TMPRSS2 on the plasma membrane174,175,176. These host binding receptors and processing enzymes provide possible therapeutic targets that are under exploration. Once in the cytoplasm, the viral RNA is released, a portion of the genome is translated and the resulting polyproteins undergo protease cleavage, yielding non-structural proteins that form components of the RNA replication complex, including RNA-dependent RNA polymerase217,218. Impairment of the viral proteases has demonstrated the value of these antigens as therapeutic targets. The RNA genome replication provides an avenue of therapeutic intervention via nucleoside analogues. ER, endoplasmic reticulum. c, COVID-19-associated immunomodulatory effects. Cell death and viral infection will drive a proinflammatory cytokine release in the host, leading to an inflammatory cycle and possible cytokine storm. Further disease progression results in tissue damage and ultimately the development of ARDS219,220. Reducing inflammation and targeting proinflammatory mediators is a promising approach to treat later stages of COVID-19 disease. WBCs, white blood cells. Panels adapted from: b, ref. 221 under a Creative Commons license CC BY 4.0; c, ref. 222 under a Creative Commons license CC BY 4.0.
Treatment early in the pandemic
The standard of care for patients was primarily supportive care at first. Azithromycin and/or hydroxychloroquine were administered as possible treatment for COVID-19 and antibiotics were utilized for possible bacterial co-infections in hospitalized patients, despite unproven efficacy. A literature review that included nine studies published by early 2020 concluded that 72% of hospitalized patients with COVID-19 were empirically treated with antimicrobials but only 8% were diagnosed with a bacterial or fungal co-infection43. Azithromycin has in vitro antiviral activity possibly limiting viral replication44, and hydroxychloroquine has been shown to have antiviral activity against SARS-CoV-2 in vitro and block viral entry45; when combined in vitro, they were observed to have a synergistic effect on SARS-CoV-2 infection46. However, hydroxychloroquine and azithromycin were not effective in clinical trials and are not recommended for the treatment or prevention of COVID-19 by the National Institutes of Health (NIH) Treatment Guidelines Panel47. These combinations were routinely used early in the pandemic. With later data showing that these regimens yield no clinical benefit, it provides a caution as to how limited in vitro data can be misinterpreted. Similarly, no specific recommendations for empiric antimicrobials are supported by the NIH COVID-19 Treatment Guidelines Panel due to insufficient data. Vitamins and mineral supplements—including zinc, which at high concentrations is thought to impair replication in some RNA viruses—were also studied early in the pandemic. In a multicenter retrospective cohort study that evaluated the addition of zinc to hydroxychloroquine in hospitalized patients, no benefit was shown48, and it is not currently recommended for the treatment of patients with COVID-19.
As the clinical manifestations, pathogenesis and complications of COVID-19 infection were better characterized, clinicians focused on triaging and monitoring patients at high risk for progression of respiratory symptoms. The NIH COVID-19 Treatment Guidelines Panel recommends providing timely supplemental oxygenation, as appropriate, and the use of high-flow nasal cannula in patients with acute hypoxemic respiratory failure is preferred over non-invasive positive pressure ventilation due to better outcomes, including a reduced rate of intubation49. When the high incidence of venous thrombo-embolic events was recognized, prophylactic anticoagulation was incorporated into the guidelines for patients hospitalized with COVID-19. Clinical recommendations continued to evolve as more data accumulated on what worked, in the absence of effective therapies.
Therapeutics development in a pandemic
Initial therapeutic development activities focused on repurposing licensed drugs (antiviral and immunomodulating) or agents that had existing clinical or non-clinical toxicology data, monoclonal antibodies that targeted the SARS-CoV-2 Wuhan variant spike protein and de novo drug development. Higher priority was given to drug repurposing and monoclonal antibodies due to the time that it takes to identify a new molecule, complete Investigational New Drug-enabling studies and produce the Good Manufacturing Practice clinical material in initial efforts to develop drugs against COVID-19.
Drug repurposing
Protease inhibitors, lopinavir–ritonavir and nelfinavir, and the antimalarial drug chloroquine had in vitro and/or in vivo antiviral activity for SARS or MERS coronaviruses50,51,52. However, in vitro anti-SARS activity of lopinavir and chloroquine/hydroxychloroquine has failed to translate into clinical benefit in COVID-19 clinical trials53,54. This might reflect a failure of the initial in vitro testing to distinguish between a true antiviral effect and cellular toxicity or could be due to the limitations of the cell models utilized55,56,57. Notably, these agents also failed to demonstrate a compelling antiviral effect using in vivo animal models, suggesting that in vivo models are a necessary intermediate step in the evaluation of repurposed agents before resource-intensive clinical evaluation58. During the early HIV pandemic drug development focused on in vitro activity and demonstrating that the appropriate level of drug could be detected in a patient59. Although repurposing screens can be accelerated towards clinical evaluation, screening and evaluation tools must be robust if we are to avoid the unnecessary use of resources in the midst of a pandemic.
Early repurposing efforts also included the assessment of antiviral agents in pre-licensure evaluation for the treatment of other viral infections. Such efforts focused on nucleotide prodrugs. One such prodrug, remdesivir (also known as GS-5734), demonstrated antiviral activity against various RNA viruses—including yellow fever, Dengue type 2, influenza A, parainfluenza 3 and SARS viruses60,61—and had been tested for activity against Ebola in a randomized clinal trial, although it did not meet mortality-related efficacy endpoints62. Other prodrugs include AT-527, active against hepatitis C virus (HCV)63; favipiravir, approved for pandemic influenza in Japan64; and molnupiravir (also known as MK-4482, EIDD-2801 and EIDD-1931-isopropyl ester), whose active component, N-hydroxycytidine (also known as EIDD-1931), was initially discovered as a bacterial mutagen65,66,67 but subsequently demonstrated activity against influenza virus68,69, respiratory syncytial virus70 and various coronaviruses71.
With pandemic preparedness in mind, several coronavirus academic laboratories had already generated an Investigational New Drug application to investigate the activity of molnupiravir against Venezuelan equine encephalitis virus72. Another repurposed drug for COVID-19 is nitazoxanide (Alinia, Romark Laboratories), the US Food and Drug Administration (FDA)-approved broad-spectrum thiazolide agent that has been used to treat various helminthic, protozoal and viral infections (including influenza virus, rotavirus, norovirus, and hepatitis B and C viruses)73. In a recent review, nitazoxanide’s mechanism of antiviral activity against SARS-CoV-2 was proposed to include inhibition of viral entry and multiplication74. Ivermectin is an FDA-approved antiparasitic drug that was also repurposed after in vitro data indicated that it inhibited SARS-CoV-2 viral replication. It was also postulated to inhibit viral entry by preventing the attachment of the spike protein to the human cell membrane75,76. Subsequent clinical studies failed to demonstrate any clinical benefit with ivermectin77,78.
Remdesivir (Veklury; developed by Gilead Sciences) is administered by intravenous infusion and is the only agent resulting from drug repurposing campaigns in early 2020 to achieve success in the first year of the pandemic, receiving full approval from the FDA in October 2020 for the treatment of COVID-19 in patients requiring hospitalization, just nine months after the first reports of COVID-19 in the United States79. Such rapid progression of remdesivir was enabled by pre-clinical characterization of in vitro and in vivo activity of the product against related coronaviruses, pharmacokinetic evaluation in healthy human volunteers and safety data acquired in an unsuccessful clinical trial for Ebola61, all of which occurred before the onset of the current SARS-CoV-2 pandemic. It should be noted that the WHO SOLIDARITY trial was unable to reproduce the positive findings from the remdesivir ACTT-1 trial54,80. In a Phase 3 study (DisCoVeRy, NCT04315948) conducted in hospitalized patients with COVID-19 receiving standard of care with remdesivir compared with standard of care alone, no clinical benefit with remdesivir was observed in patients who were symptomatic for more than 7 d (ref. 81). In addition, a retrospective study on the impact of timing of remdesivir treatment and mortality benefit showed that patients with moderate-to-severe COVID-19 with treatment initiation ≤9 d from the onset of symptoms compared with >9 d from symptom onset had significantly lower odds of death (odds ratio = 0.43; 95% confidence interval, 0.25–0.75; P = 0.003)82. This may be driven by the pathology of SARS-CoV-2, where the opportunity to mitigate against severe disease with an antiviral may diminish as the disease progresses from one primarily attributable to viral damage to host cells to one attributable to host inflammatory response, similar to what is observed for anti-influenza treatments that need to be administered early in the course of disease to be effective29. This is supported by a Phase 3 study (PINETREE, NCT04501952) that was conducted with patients who were either outpatients or residing in skilled nursing facilities. In this study, remdesevir given within 7 d of symptom onset reduced the risk of hospitalization by 87% compared with placebo in patients at high risk of severe disease83. Following these findings, remdesivir’s approval was expanded to high-risk non-hospitalized patients84. Clinical development of inhaled remdesivir85 in a Phase 1b/2a study (NCT04539262) as well as pre-clinical development of an oral prodrug of remdesivir (GS-621763)86 are ongoing. In addition, Shanghai Junshi Biosciences has advanced the oral remdesivir derivate VV116, which has completed a non-inferiority observer-blinded randomized Phase 3 trial (NCT05341609) versus Paxlovid (see the section ‘Protease inhibitors’) in seven hospitals in China for the treatment of mild-to-moderate COVID-19 (ref. 87). VV116 was recently approved for marketing in China88.
Other repurposed drugs have also been clinically assessed for activity against COVID-19. AT-527 (Atea Pharmaceuticals), an HCV compound, was evaluated in a Phase 2 randomized trial in non-hospitalized adult patients with mild or moderate COVID-19 (NCT04709835). Treatment with AT-527 did not result in a clear reduction in SARS-CoV-2 viral load compared with the placebo (primary endpoint) in the overall population of patients with mild or moderate COVID-19 (ref. 89). Further Phase 2 evaluation of safety and efficacy of AT-527 in hospitalized patients with moderate COVID-19 is ongoing (NCT04396106). Clinical trials were also conducted in several countries to evaluate the safety and efficacy of favipiravir, an orally administered anti-influenza drug90. For example, in a randomized, double-blinded, multicenter and placebo-controlled trial conducted in Saudi Arabia in adult patients with mild COVID-19, 1,800 mg favipiravir administered twice on day 1, followed by 800 mg twice daily (n = 112) for a total of 5–7 d did not reduce time to viral clearance within 15 d of starting the treatment, thus demonstrating lack of efficacy for favipiravir therapy91. Nitazoxanide (Alinia, Romark Laboratories) was evaluated in a multicenter randomized clinical trial in patients with mild COVID-19. In comparison to the placebo, the drug did not have an effect on symptom resolution after 5 d of therapy, although it significantly reduced viral load (P = 0.006)92. At present, the COVID-19 Treatment Guidelines Panel recommends against the use of nitazoxanide for the treatment of COVID-19, except in a clinical trial93. In a randomized Phase 3 trial, three repurposed drugs—ivermectin, metformin and fluvoxamine—were evaluated for the early treatment of COVID-19 in adults who were either overweight or obese. None of the three medications had a significant effect on the prevention of a serious outcome defined as hypoxaemia, emergency department visit, hospitalization or death94.
The pre-pandemic development of molnupiravir (also known as MK-4482; Lagevrio, Merck) as a treatment for coronaviruses was slowed by mutagenic potential65,67,95. This oral compound entered clinical studies in healthy individuals in April 2020 (NCT04392219)96 and progressed to patients in June 2020 (NCT04405570)97. Pivotal efficacy studies were initiated in October 2020 in hospitalized (NCT04575584) and non-hospitalized (NCT04575597) adults with COVID-19. Following an interim analysis, it was concluded that molnupiravir was unlikely to provide clinical benefit in hospitalized patients and the inpatient MOVe-IN study was discontinued98,99. At the time of hospitalization patients are usually in the later stages of disease (Stages 2 and 3) and further out from disease onset, thus limiting the impact of antivirals on disease course. In contrast, the outpatient MOVe-OUT study demonstrated that earlier treatment (≤5 d) decreased the risk of hospitalization or death in at-risk patients when compared with placebo97. Molnupiravir achieved an Emergency Use Authorization (EUA) in the United States on 23 December 2021 for the treatment of mild-to-moderate COVID-19 in adults at high risk for progression to severe COVID-19, including hospitalization or death24. However, a recently published study conducted in the UK found that molnupiravir did not reduce the frequency of COVID-19–associated hospitalizations or death of high-risk vaccinated adults (≥50 yr or 18–49 yr with a high-risk condition)100. In addition, in all immunocompromised patients treated with molnupiravir who did not clear SARS-CoV-2 infection after treatment, SARS-CoV-2 rapidly accrued new mutations in the spike protein, including nonsynonymous mutations that altered the amino-acid sequence, which were persistent or even fixed in the virus population101. The authors suggest that treatment with molnupiravir may ‘supercharge’ viral evolution in immunocompromised patients.
Beyond these targeted repurposing efforts, several additional initiatives to identify candidate agents that may be repurposed for use in treating COVID-19 have been undertaken. On 10 March 2020 the Bill and Melinda Gates Foundation announced the launch of the Therapeutics Accelerator, supported by US$125 million from the Wellcome Trust, Mastercard and the Bill and Melinda Gates Foundation, with an additional US$250 million subsequently committed by a variety of individuals and institutions102. The Accelerator adopted a three-pronged approach to identifying candidate compounds for COVID-19: (1) testing approved drugs, (2) screening libraries of agents with confirmed safety data and (3) evaluating novel agents (including antibodies) for anti-SARS-CoV-2 activity. As part of this effort, various collaborations were established between Calibr, the drug development division of Scripps Research, and a number of outside research teams to screen the ReFRAME drug repurposing collection (comprised of >14,000 compounds that have been approved by the FDA for other diseases or have been extensively tested for human safety) for agents active against SARS-CoV-2 (ref. 103). Initial publications of the ReFRAME library screening report the identification of numerous agents for which the effective concentrations against SARS-CoV-2 can probably be attained in patients, of which apilimod and camostat mesilate seem to have undergone the most extensive follow-on testing. Apilimod, a PIKfyve kinase inhibitor that was originally developed for the treatment of autoimmune conditions104, is thought to prevent intracellular trafficking of the virus following entry into host cells and its evaluation has subsequently begun in a clinical trial in non-hospitalized adults with confirmed COVID-19 infection (NCT04446377). Verge Genomics has also repositioned their selective oral PIKfyve inhibitor, originally developed for amyotrophic lateral sclerosis, for COVID-19 clinical studies105. Camostat mesilate, an inhibitor of the human serine protease transmembrane protease serine 2 (TMPRSS2) thought to be required for entry of the virus into host cells, is approved in Japan for the treatment of chronic pancreatitis and postoperative reflux esophagitis106. Camostat mesylate was investigated in multiple clinical trials in both hospitalized and non-hospitalized adults with confirmed COVID-19 infection107,108,109,110,111.
In addition to the ReFRAME library screening, numerous other efforts to identify potential candidates for repurposing have been undertaken, including in silico docking, biochemical screens, cell-based screens and in vivo testing58. Given the number of potential candidates identified, additional testing in in vitro and/or in vivo models with high confidence in translatability to clinical efficacy will be an important step in prioritizing agents for further clinical evaluation.
Immunotherapeutics
In emergency phases of disease outbreaks, passive transfusion of convalescent blood products has often been used and a number of case reports in early 2020 reported evidence of benefit among patients with COVID-19 who received convalescent plasma112. However, challenges exist with this therapy—these include collection, pathogen inactivation, standardization of dosing and the assumption that the donor has had a robust neutralizing antibody response to the virus. Despite these limitations, plasma infusion can be administered to patients who are immunocompromised and patients who are hospitalized before symptom onset.
Rapid identification and characterization of potent neutralizing monoclonal antibodies targeting the SARS-CoV-2 spike protein is possible using high-throughput amplification, cloning, expression and functional screening of heavy- and light-chain pairs of SARS-CoV-2-specific immunoglobulins from sorted memory B cells isolated from peripheral blood mononuclear cells of donors that have strong SARS-CoV-2-neutralization activity in convalescent plasma113,114. Mono- or polyclonal antibody cocktails can be accelerated into clinical testing, as observed to date with two monoclonal antibody cocktails and two single monoclonal antibodies that received an EUA by the FDA for the treatment of COVID-19 outpatients who are at high risk for progressing to severe COVID-19 (Regeneron Pharmaceuticals, casirivimab with imdevimab (REGEN-COV)31,115; Eli Lilly, bamlanivimab with etesevimab30,116; GSK, sotrovimab (Xevudy)117 and Eli Lilly, bebtelovimab118). Another monoclonal antibody cocktail, tixagevimab with cilgavimab (Evusheld, AstraZeneca), received an EUA for pre-exposure prophylaxis for people who are at high risk of severe COVID-19 outcomes21,119. As SARS-CoV-2 continued to evolve, changes in the spike protein led to EUAs being withdrawn for all of the monoclonal antibodies—including casirivimab with imdevimab, bamlanivimab with etesevimab, sotrovimab and bebtelovimab—due to loss of efficacy21,22.
Anti-inflammatory and immunomodulatory therapies
Targeting host responses using anti-inflammatory and immunomodulating drugs may be useful in later stages of COVID-19. For example, treatment with corticosteroids (dexamethasone) reduced mortality within a 28-d period in patients hospitalized with COVID-19 who received oxygen supplementation or required invasive mechanical ventilation compared with those who received standard of care (29.3% versus 41.4%; rate ratio, 0.64; 95% confidence interval, 0.51–0.81)120. The treatment, however, could potentially be deleterious for patients with active viraemia120. An orally administered immunomodulating inhibitor of Janus kinase (JAK), baricitinib (Olumiant, Eli Lilly), in combination with remdesivir improved outcomes for hospitalized patients with late-stage disease, reducing the time to recovery to within 29 d after initiating treatment compared with placebo with remdesivir, resulting in an EUA121,122. In combination, corticosteroids and baricitinib are recommended by the WHO for patients with severe or critical COVID-19 (refs. 123,124). In May 2022 baricitinib was approved by the FDA for the treatment of COVID-19 in hospitalized adults requiring various degrees of oxygen support125,126. In December 2022, Eli Lilly withdrew its EU marketing authorization application for the use of baricitinib in the treatment of hospitalized COVID-19 patients who require supplemental oxygen127.
Interleukin (IL) inhibitors have also been investigated, with an IL-1 inhibitor (IL-1R antagonist, anakinra (Kineret, Swedish Orphan Biovitrum)), showing a significant benefit against mortality (hazard ratio, 0.450; 95% confidence interval, 0.204–0.990; P = 0.047) in hospitalized patients with COVID-19, respiratory insufficiency and evidence of inflammation, whereas IL-6 inhibitors (monoclonal antibodies to IL-6 and IL-6 receptor) did not have an effect in this assessment (hazard ratio, 0.900; 95% confidence interval, 0.412–1.966; P = 0.79)128. However, the study design was an observational cohort study that included only a small number of patients where 62 received an IL-1 inhibitor and 55 received an IL-6 inhibitor. In a large randomized, controlled, open-label, platform ReCOVERY clinical trial in hospitalized patients with COVID-19 that had hypoxia and systemic inflammation, a monoclonal antibody to IL-6, tocilizumab (Actemra, Genentech), significantly improved the 28-d survival (P = 0.0028) and other clinical outcomes129. Tocilizumab received an EUA in June 2021 for the treatment of hospitalized patients receiving corticosteroids and supplemental oxygen130.
Initial promising results were also seen for the antiviral cytokine candidate peginterferon lambda. Type III interferon lambdas are a recently discovered class of interferons that stimulate antiviral immune responses that are largely exerted at epithelial surfaces such as the respiratory and gastrointestinal tracts131. Due to a less ubiquitous receptor expression, type III interferons as therapeutic agents are more tolerable than type I interferons131. Peginterferon lambda is an investigational therapeutic agent that was originally developed for the treatment of HCV132 and is currently being developed by Eiger BioPharmaceuticals for the treatment of COVID-19. In a randomized placebo-controlled Phase 3 study in non-hospitalized adult patients with COVID-19 at high risk of progressing to severe illness, a single subcutaneous dose of peginterferon lambda reduced the risk of hospitalization or emergency room visits by 50% and the risk of death by 60% (ref. 133).
Another agent is sabizabulin (also known as Veru-111), a bis-indole compound undergoing development by Veru Inc. that inhibits tubulin polymerization, blocking intracellular virus trafficking, and exhibits broad anti-inflammatory and antiviral activity in pre-clinical studies134. In a randomized Phase 3 study in hospitalized patients with moderate-to-severe COVID-19 at high risk for ARDS, an interim analysis demonstrated a 55% reduction in the COVID-19-related risk of death following oral treatment with 9 mg sabizabulin compared with placebo134. The FDA granted Fast Track designation to the sabizabulin COVID-19 clinical programme in January 2022 (ref. 135).
Antivirals for SARS-CoV-2
With the exception of monoclonal antibodies and the intravenous treatment remdesivir, no new antiviral agents were available 12 months after the pandemic was declared. Several antivirals, including protease inhibitors and spike protein inhibitors, have subsequently advanced to clinical trials.
Protease inhibitors
One notable therapeutic target is the main protease (Mpro, also known as 3C-like protease, 3CL)136. Mpro is responsible for hydrolysis of the viral replicase polyproteins pp1a and pp1ab to produce functional proteins during replication. Mpro is an attractive target as the sequence is highly conserved across MERS-CoV, SARS-CoV-1 and SARS-CoV-2, with a cysteine-histidine dyad active site that has no human homologue136. The first protease inhibitors were developed to treat chronic infections associated with HIV and HCV, and have proven to be safe and effective antiviral treatments137,138. Pfizer had two protease inhibitors, PF-07304814 and nirmatrelvir (also known as PF-07301332; the active Mpro inhibitor of Paxlovid; Fig. 2a), that were clinically evaluated to potentially treat COVID-19. Both PF-07304814 and nirmatrelvir are peptidomimetic covalent inhibitors that mimic the peptide substrate of the cysteine proteases. Peptidomimetics are designed to mitigate the poor absorption and metabolic stability of peptide drugs, which are usually driven by proteolysis that can limit bioavailability. Intravenous dosing can be used to address the challenges and is a common option used with hospital-based antiviral agents. PF-07304814, which is administered through the intravenous route, is a prodrug of a compound identified in 2003 during a SARS-CoV-1 discovery effort139. PF-07304814 was found to be a specific inhibitor of Mpro with broad in vitro activity across different SARS-CoV-2 variants and other coronaviruses tested140. Unlike Merck’s molnupiravir, a toxicology package had not been completed on this compound before the pandemic. Between January 2020 and August 2020, the prodrug was developed, scaled up and evaluated in toxicology studies to permit an open Investigational New Drug in August to initiate testing in hospitalized patients (NCT04535167). PF-07304814 required being administered as a continuous intravenous infusion for 5 d, which made clinical studies challenging. In addition, protease inhibitors were expected to have the greatest benefit during the acute phase of disease, before hospitalization. The development of PF-07304814 was halted in early 2022 (ref. 141).
Nirmatrelvir was a new Mpro inhibitor that was identified by Pfizer as part of a COVID-19 discovery programme initiated early in 2020, with an approach taken to attempt to maximize the oral bioavailability142. This novel oral candidate was discovered in July 2020 and underwent pre-clinical toxicology studies and scale-up during the latter part of 2020 to enable clinical studies to be initiated in March 2021 (NCT04756531). Although nirmatrelvir had increased oral bioavailability, the majority of its metabolites are from cytochrome P450 (CYP)-mediated oxidations143. Co-dosing with the CYP-3 inhibitor ritonavir helped maximize the oral exposures of nirmatrelvir to median levels of 5× the 90% effective concentration from in vitro assays, and a Phase 2/3 pivotal study, Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR; NCT04960202) was initiated in July 2021 (ref. 142). In EPIC-HR, risk reductions of 88–89% at preventing hospitalization and 100% at preventing death were demonstrated in unvaccinated patients at high risk of severe disease32. This study led to Paxlovid (nirmatrelvir co-dosed with ritonavir) being granted an EUA on 22 December 2021 for the treatment of high-risk outpatients with mild-to-moderate COVID-19 (ref. 23).
Two other Phase 2/3 efficacy trials of nirmatrelvir–ritonavir were also conducted: Evaluation of Protease Inhibition for COVID-19 in Post-Exposure Prophylaxis (EPIC-PEP; NCT05047601) and Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients (EPIC-SR; NCT05011513). EPIC-PEP, initiated in September 2021, assessed whether individuals exposed to patients with COVID-19 could be protected from acquiring symptomatic COVID-19. Although the study did not meet its primary endpoint, compared with placebo, risk reductions of 32% and 37% were observed in adults who received Paxlovid for 5 and 10 d, respectively, to prevent infection144. EPIC-SR, initiated in August 2021, assessed the efficacy and safety of nirmatrelvir–ritonavir in non-hospitalized symptomatic adults with COVID-19 at low risk of progressing to severe illness. Although the novel primary endpoint of self-reported sustained alleviation of all symptoms for four consecutive days was not met, analyses showed a non-significant relative risk reduction in hospitalizations and death compared with the placebo group—51% for all patients enrolled through December 2021 and 57% for vaccinated patients with at least one risk factor for severe COVID-19—providing support for the efficacy data observed in the EPIC-HR study. In addition, a nominally significant 62% decrease in COVID-19-related medical visits per day across all patients, relative to the placebo group, was observed. Due to a very low rate of hospitalization or death observed in the standard-risk patient population, enrollment was ceased and the study was terminated145.
Similar to PF-07304814, nirmatrelvir has the potential for broad coronavirus activity with good margins in pre-clinical safety studies and a clean genotoxicity profile, and displays potent in vitro inhibitory activity against SARS-CoV-2 variants of concern, including Omicron146,147,148,149,150. The potential for resistance has also been evaluated; the non-synonymous mutation rate (substitution per residue per year) was estimated to be lower for Mpro (2.43 × 10−4) compared with the RNA-dependent RNA polymerase (9.18 × 10−4)151. Early in vitro analysis using sequential drug passaging has demonstrated that resistance to nirmatrelvir can occur152,153,154, however, at this time no clinical SARS-CoV-2 resistance to nirmatrelvir has been reported155. The pharmacokinetic profile of nirmatrelvir is improved by the co-dosing of ritonavir; however, ritonavir is a strong CYP-3 inhibitor and may increase the concentrations of certain concomitant medications due to drug–drug interactions. A thorough review of the medication list of the patient is needed before initiating nirmatrelvir–ritonavir, with possible dose adjustment required of the concomitant medication or selection of an alternative COVID-19 therapeutic should there be concern for a drug–drug interaction that might lead to potentially serious and/or life-threatening adverse events156,157. Next-generation peptidomimetic protease inhibitors have the potential to be further optimized to improve pharmacokinetics to remove the need to co-dose ritonavir.
In July 2021 Shionogi & Co. announced the initiation of a Phase 1 clinical trial of the oral drug S-217622 (also known as ensitrelvir; Fig. 2b) in Japan158. S-217622 is claimed to be a selective inhibitor of Mpro that was demonstrated to reduce viral load in pre-clinical in vivo studies of SARS-CoV-2-infected animals159. Subsequently, Shionogi & Co. initiated a Phase 2/3 trial (jRCT2031210350) to evaluate the efficacy and safety of a 5-d treatment with S-217622 compared with placebo in patients with asymptomatic and mild COVID-19 infections160. Results were recently released from a Phase 2b study where S-217622-treated patients had shortened duration of shedding of infectious virus and experienced a significant reduction in viral RNA on days 2, 4, 6 and 9 compared with placebo161. However, there was no significant difference in the total score of the 12 symptoms between the two groups. In addition, Todos Medical and NLC Pharma initiated Phase 2 trials with Tollovir (NLC-V-01), a Mpro inhibitor, at the Shaare Zedek Medical Center in Jerusalem, Israel162. A double-blind, placebo-controlled and randomised Phase 2 trial evaluating the safety and efficacy of Tollovir in hospitalized patients reported positive results163. The study included small numbers of participants (11 Tollovir-treated and 9 placebo-treated) yet reported a shorter time to clinical improvement and decreased incidence of death (0% (0/11) versus 22% (2/9))164. Pardes Biosciences is developing PBI-0451 (Fig. 2c), an orally administered coronavirus Mpro inhibitor that has demonstrated in vitro activity against multiple coronaviruses, including SARS-CoV-2 and variants of concern such as Delta and some Omicron lineages, as well as other human coronaviruses of pandemic potential (SARS and MERS) and common cold-related coronavirus strains (OC43 and 229E)165. PBI-0451 has shown favourable safety and tolerability in a Phase 1 trial (NCT05011812), and the antiviral activity, safety and efficacy of this investigational compound, compared with placebo, are being evaluated in a Phase 2 double-blind randomized study in non-hospitalized symptomatic adults with COVID-19 (NCT05543707)166. In March 2022 Enanta Pharmaceuticals received an FDA fast track designation for EDP-235, a novel oral Mpro inhibitor, which has now completed Phase 1 trial (NCT05246878)167. Aligos Therapeutics in collaboration with KU Leuven have also announced the selection of the drug candidate ALG-097558 (a potent oral Mpro inhibitor) for the treatment and prevention of COVID-19, planning to file a Phase 1 trial application in the second half of 2022 (ref. 168).
Spike protein inhibitors
Mono- or multi-specific engineered designed ankyrin repeat proteins (DARPins)169, similar to monoclonal antibodies in their ability to bind antigen, are also in development as a potential antiviral immunotherapy for COVID-19. The DARPin candidate molecule for COVID-19 ensovibep (also known as MP0420, Novartis) comprises three individual domains, each highly neutralizing to SARS-CoV-2, and effectively blocks the receptor-binding domain of the SARS-CoV-2 spike protein due to cooperative binding170. This design ensures strong neutralization, even in the presence of mutations of the spike protein, and limits the development of escape mutants. An ability of ensovibep to protect against SARS-CoV-2 variants of concern, including Omicron, in a pre-clinical model has been reported171. In a Phase 2 randomized study in non-hospitalized adult patients with COVID-19 (NCT04828161), a 78% reduction in risk of hospitalization and/or emergency room visits related to COVID-19, or death, compared with placebo was observed following a single intravenous dose of ensovibep. A significant reduction in the viral load over 8 d compared with placebo was also observed172.
Early phase pre-clinical therapeutic development
For the early viral infection stages, cell entry and endosomal trafficking are attractive points of intervention with the primary focus being on human host targets. Given that angiotensin-converting enzyme 2 (ACE2) is the receptor to SARS-CoV-2, it makes it a compelling target for blocking cell entry during infection. Profiling of inhibitors of ACE2 catalytic enzymatic function has demonstrated that the catalytic activity of ACE2 and binding event of SARS-CoV-2 to ACE2 are independent. The vast number of ACE2 catalytic inhibitors have not been shown to block an in vitro infection173. Alternatively, the use of recombinant ACE2 as a decoy strategy to block SARS-CoV-2 cell entry is currently in clinical trials by Apeiron Biologics (NCT04335136). Further opportunities to target ACE2 internalization or shedding from the cell membrane, in addition to allosteric small molecules that could disrupt the SARS-COV-2 spike protein-binding site, could be a future avenue for drug discovery.
In conjunction with binding SARS-CoV-2 to ACE2, human host proteases that process the spike protein post binding to ACE2 are required for viral entry. It has been demonstrated that the SARS-CoV-2 spike glycoprotein is cleaved by the host serine protease TMPRSS2 and proprotein convertase furin174,175,176. As discussed earlier, several repurposed approved human protease inhibitors have entered human trials107,108,177,178,179. If successful in the clinical studies, future research and development of more potent selective inhibitors targeting specific human proteases for the spike glycoprotein may be warranted; however, off-target activity with human proteins is something that will need to be monitored closely.
Additional targets recently discovered to block the viral entry of SARS-CoV-2 into lung epithelial cells were shown to regulate endocytosis, including AP2-associated protein kinase 1 (AAK1)180. AAK1 is a known regulator in AT2 alveolar epithelial cells and has been proposed as a target to block virus entry into cells180. Baricitinib, a JAK 1 and 2 inhibitor, was shown to also bind to AAK1 through screening of approved drugs180. Baricitinib has already been evaluated in multiple trials, primarily for anti-inflammatory pharmacology, but selective targeting of AAK1 to prevent virus entering cells may be an additional strategy for possible intervention of an early infection181.
Viral proteases are of a particular interest given that they are required for viral replication and, if targeted, selectivity would spare directly targeting the host. In addition to Mpro agents in clinical trials from Pfizer and Shionogi & Co., additional antiviral Mpro inhibitors are in pre-clinical development. The COVID Moonshot international consortium, initiated early in the pandemic, is focused on the discovery of antiviral Mpro inhibitors using the novel open-science crowdsourced approach182. Beyond Mpro inhibition, a second viral protease required for SARS-CoV-2 replication is papain-like protease (PLpro). PLpro is required by SARS-CoV-2 to generate the replicase complex but also acts as a protease and cleaves both human ubiquitin and ISG15, which are regulators of the host innate immune response for viral evasion183. This dual pharmacology could address viral replication at the initial stages as well as possibly at the later inflammatory stages. There are no publicly disclosed clinical agents for PLpro at present.
Outlook
Although a coronavirus pandemic was perhaps predictable184, the world was woefully unprepared3. Despite this, vaccine development and initial deployment was achieved in under 12 months, a process that usually takes tens of years185. Symptomatic infections can occur in populations that are vaccinated, enabling ongoing community transmission186,187. Antiviral discovery and development therefore still have an important role to play in the control and mitigation of COVID-19 infection and transmission. FDA-authorized or FDA-approved COVID-19 therapeutics are summarized in Table 1. Advantages and disadvantages of COVID-19 antiviral drugs are discussed in Box 1. In March 2022 the WHO provided a Strategic Preparedness and Response Plan to end the acute phase of the pandemic, requiring two objectives to be attained: first, reduction in the incidence of SARS-CoV-2 infections and second, prevention, diagnosis and treatment of COVID-19 (ref. 188). Over the course of the pandemic we have moved closer to achieving these goals, the management of hospitalized patients improved, therapeutics administration expanded in the in- and outpatient settings, and vaccines were realized. Drug development progressed rapidly to clinic during the pandemic, owing in part to public–private partnerships (for example, NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines, ACTIV), substantial investments made in needed research infrastructure (for example, biosafety level 3 facilities), advances made in the use of the ReFRAME library to identify molecules and innovative ways to accelerate clinical trials (flexibility of protocols), to name a few.
From a drug development perspective, we have learned that we can design and pre-clinically evaluate new molecules quickly. The initial focus on screening the FDA-approved drugs, or agents that are close to or currently in clinical trials, with the goal of repurposing towards COVID-19 treatments, has informed possible future avenues for discovery. Potential targets to treat early stage infection or prophylactic treatment following potential exposure for both virus and host show promise. In later stages, primary efforts have focused on host immunity targets to control cytokine storm effects and inflammation, although treatments to lower viral load continue to be of interest as well, particularly for immunocompromised patients.
We still have a lot to learn about COVID-19 pathogenesis and treatment. Although it is caused by an acute-phase virus, where antiviral treatment needs to be initiated early, its deleterious impact can result in severe disease and persistence of chronic symptoms for weeks or even months, despite no conclusive evidence of a viral reservoir. As diagnostic tests became more available, a phenomenon known as COVID rebound was reported, where the patient could test positive for the virus and/or have disease symptoms a few days after clearing the virus189,190. The aetiology of viral load rebound is unknown and in the general untreated outpatient population, viral load rebound has been observed in up to 12% of cases40. Only a smaller proportion of those with viral load rebound – 1-2% – also reported symptom rebound following initial improvement40. Viral load rebound has also been observed in patients treated with Paxlovid and molnupiravir191. In a retrospective analysis of the EPIC-HR (NCT04960202) population and a small case series of patients treated with nirmatrelvir–ritonavir, viral load rebound was not associated with the development of resistance192,193. Although these rebounds have not been associated with serious outcomes, it highlights how hard it is to control the transmission of COVID-19 for patients with prolonged detectable virus. As with viral rebound, the aetiology, manifestations and possible preventative measures and/or treatment of post-COVID conditions are not fully understood. These include long COVID, also known as post-acute sequelae of SARS-CoV-2 (PASC) and post-COVID-19 syndrome. This is a growing area of unmet medical need, with an estimated global prevalence of 37 and 32% of those with COVID-19 having experienced persistent symptoms due to COVID-19 at 30 and 90 d, respectively194. Extensive research efforts are ongoing to both classify the disease—which may actually be several syndromes—and determine the cause. Early hypotheses include residual viraemia, reactivated latent viraemia from other viruses such as Epstein–Barr virus and the activation of autoantibodies195,196,197. These studies have shown that autoantibodies increase over time during COVID-19 infection. Studies of patients with existing autoimmune diseases may also shed more light on the pathophysiology of long COVID. A study in patients with established primary Sjögren’s syndrome and recent COVID-19 infection identified that 29% of the study population had symptoms past 12 weeks, with raised levels of lactate dehydrogenase and C-reactive protein, supplemental oxygen and hospital admission being associated with a higher risk (odds ratio >5) of developing long COVID198. There is also hope in the autoimmune disorder community that the increased investment into long COVID research may find improved treatments for them. In February 2022 the US government committed over one billion dollars for long COVID research, for which its flagship programme RECOVER (https://recovercovid.org/) aims to understand, prevent and treat long-term health effects related to COVID.
Despite the severity of COVID-19 infections and the uncertainties of the long-term effects of PASC, three years into the COVID-19 pandemic we seem to be in a state of COVID-19 fatigue. Public health measures designed to prevent the spread of disease have all but stopped and fatigue is also making it harder to estimate the actual overall burden, as many people do not test themselves and those who do, do not always report it. As of 15 February 2023, although 79.0% of the US adult population (≥18 yr) had completed the primary vaccine series, only 19.3% had received the updated bivalent booster dose199. The vaccination rates were higher in the older adults (age ≥65 yr) for both the primary series (94.2%) and the bivalent booster dose (41.0%)199. Furthermore, in the period between 3 October 2021 and 24 December 2022, among unvaccinated people aged ≥12 yrs in the US, there was an approximately 14-fold increased risk of dying from COVID-19 compared with people who received the bivalent booster vaccine200. This is compounded by the early monoclonal antibodies losing efficacy as the virus mutates. This emphasizes the need for safe and effective COVID-19 therapies that have different mechanisms of action, can be deployed across the population to provide treatment options and can reduce the potential for resistance development.
Although remdesivir, nirmatrelvir–ritonavir and molnupiravir were developed with unprecedented speed, we need to find ways to further accelerate small molecule development from the approximately 2 yr it took to develop antivirals that could be administered by the patient at home to circumvent serious disease. Although we are unable to predict the culprit of the next pandemic, to enable rapid development of therapeutics, we would need small molecules identified that have already progressed through early pre-clinical and clinical evaluation, similar to the readiness by which remdesivir was positioned at the outset of the pandemic. Ideally, these candidates would mechanistically be able to target the conserved region of the pathogen and not be easily rendered ineffective across strains. It would behove us to continue to use infrastructure developed in the COVID-19 pandemic to advance development of antivirals for other pathogens in preparation for the next pandemic.
References
WHO Coronavirus (COVID-19) Dashboard (WHO, accessed 22 February 2023); https://covid19.who.int/
Cui, J., Li, F. & Shi, Z. L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 17, 181–192 (2019).
Swerdlow, D. L. & Finelli, L. Preparation for possible sustained transmission of 2019 novel coronavirus: lessons from previous epidemics. JAMA 323, 1129–1130 (2020).
The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly 2, 113–122 (2020).
Wu, Z. & McGoogan, J. M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323, 1239–1242 (2020).
Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J. & Prescott, H. C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA 324, 782–793 (2020).
Clinical Spectrum of SARS-CoV-2 Infection (NIH, accessed 25 January 2023); https://go.nature.com/3LTfZhQ
Halpin, S. J. et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J. Med. Virol. 93, 1013–1022 (2020).
Huang, C. et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397, 220–232 (2021).
Stokes, E. K. et al. Coronavirus Disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb. Mortal. Wkly Rep. 69, 759–765 (2020).
Fried, M. W. et al. Patient characteristics and outcomes of 11,721 patients with COVID19 hospitalized across the United States. Clin. Infect. Dis. 72, e558–e565 (2020).
Garg, S. et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb. Mortal. Wkly Rep. 69, 458–464 (2020).
Golestaneh, L. et al. The association of race and COVID-19 mortality. eClinicalMedicine 25, 100455 (2020).
Brandén, M. et al. Residential context and COVID-19 mortality among adults aged 70 years and older in Stockholm: a population-based, observational study using individual-level data. Lancet Healthy Longev. 1, e80–e88 (2020).
Saloner, B., Parish, K., Ward, J. A., DiLaura, G. & Dolovich, S. COVID-19 cases and deaths in federal and state prisons. JAMA 324, 602–603 (2020).
Yi, S. H. et al. Characterization of COVID-19 in assisted living facilities—39 states, October 2020. MMWR Morb. Mortal. Wkly Rep. 69, 1730–1735 (2020).
Coronavirus Disease (COVID-19): Vaccines (WHO, accessed 15 December 2022); https://go.nature.com/40jSwuN
Kaplon, H. & Reichert, J. M. Antibodies to watch in 2021. MAbs 13, 1860476 (2021).
Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19 (US FDA, 9 November 2020); https://go.nature.com/3nq8aGr2020
Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 (US FDA, 21 November 2020); https://go.nature.com/3JMqFME
Almagro, J. C., Mellado-Sanchez, G., Pedraza-Escalona, M. & Perez-Tapia, S. M. Evolution of anti-SARS-CoV-2 therapeutic antibodies. Int. J. Mol. Sci. 23, 9763 (2022).
COVID-19 Monoclonal Antibodies (Centers for Medicare & Medicaid Services, accessed 21 February 2023); https://www.cms.gov/monoclonal
Pfizer Receives U.S. FDA Emergency Use Authorization for Novel COVID-19 Oral Antiviral Treatment (Pfizer, 22 December 2021); https://go.nature.com/3KdyjBk
Merck and Ridgeback’s Molnupiravir Receives U.S. FDA Emergency Use Authorization for the Treatment of High-Risk Adults With Mild to Moderate COVID-19 (MSD, 23 December 2021); https://go.nature.com/3TQLfA3
Alsuliman, T., Alasadi, L., Alkharat, B., Srour, M. & Alrstom, A. A review of potential treatments to date in COVID-19 patients according to the stage of the disease. Curr. Res. Transl. Med 68, 93–104 (2020).
Cascella, M., Rajnik, M., Cuomo, A., Dulebohn, S.C. & Di Napoli, R. Features, Evaluation and Treatment Coronavirus (COVID-19). In: StatPearls [Internet]. (Treasure Island (FL): StatPearls Publishing; 2023).
Jin, Y. et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 12, 372 (2020).
Siddiqi, H. K. & Mehra, M. R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transpl. 39, 405–407 (2020).
Gaitonde, D. Y., Moore, F. C. & Morgan, M. K. Influenza: diagnosis and treatment. Am. Fam. Physician 100, 751–758 (2019).
Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab (Eli Lilly and Company, 2022); https://go.nature.com/3zaRpBF
Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of REGEN-COV (Casirivimab and Imdevimab) (Regeneron, 2022); https://go.nature.com/3FXcvHA
Hammond, J. et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N. Engl. J. Med. 386, 1397–1408 (2022).
Jayk Bernal, A. et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N. Engl. J. Med. 386, 509–520 (2022).
Petty, L. A. & Malani, P. N. Oral antiviral medications for COVID-19. JAMA 327, 2464 (2022).
Merck and Ridgeback’s Molnupiravir, an Oral COVID-19 Antiviral Medicine, Receives First Authorization in the World (MSD, 4 November 2020); https://go.nature.com/40G4i2c
Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study (Pfizer, 5 November 2021); https://go.nature.com/40lf2mT
Bellon, M. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load kinetics in symptomatic children, adolescents, and adults. Clin. Infect. Dis. 73, e1384–e1386 (2021).
Vetter, P. et al. Daily viral kinetics and innate and adaptive immune response assessment in COVID-19: a case series. mSphere 5, e00827-20 (2020).
Kissler, S. M. et al. Viral dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated persons. N. Engl. J. Med. 385, 2489–2491 (2021).
Deo, R. et al. Symptom and viral rebound in untreated SARS-CoV-2 infection. Ann. Intern. Med. 176, 348–354 (2023).
Datta, S. D., Talwar, A. & Lee, J. T. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. JAMA 324, 2251–2252 (2020).
Nalbandian, A. et al. Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615 (2021).
Rawson, T. M. et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 71, 2459–2468 (2020).
Venditto, V. J. et al. Immunomodulatory effects of azithromycin revisited: potential applications to COVID-19. Front. Immunol. 12, 574425 (2021).
Yao, X. et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 71, 732–739 (2020).
Andreani, J. et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Micro. Pathog. 145, 104228 (2020).
Cavalcanti, A. B. et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. 383, 2041–2052 (2020).
Frontera, J. A. et al. Treatment with zinc is associated with reduced in-hospital mortality among COVID-19 patients: a multi-center cohort study. Preprint at Res Sq. https://doi.org/10.21203/rs.3.rs-94509/v1 (2020).
Ni, Y. N. et al. The effect of high-flow nasal cannula in reducing the mortality and the rate of endotracheal intubation when used before mechanical ventilation compared with conventional oxygen therapy and noninvasive positive pressure ventilation. A systematic review and meta-analysis. Am. J. Emerg. Med. 36, 226–233 (2018).
Chu, C. M. et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 59, 252–256 (2004).
Vincent, M. J. et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2, 69 (2005).
Yamamoto, N. et al. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 318, 719–725 (2004).
Self, W. H. et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA 324, 2165–2176 (2020).
World Health Organization Solidarity Trial Consortium et al. Repurposed antiviral drugs for COVID-19—interim WHO Solidarity Trial results. N. Engl. J. Med. 384, 497–511 (2020).
Hattori, S. I. et al. GRL-0920, an indole chloropyridinyl ester, completely blocks SARS-CoV-2 infection. mBio 11, e01833–01820 (2020).
Fan, J. et al. Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients. Clin. Infect. Dis. 71, 3232–3236 (2020).
Yuan, Z. et al. Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture. Commun. Biol. 5, 958 (2022).
Watashi, K. Identifying and repurposing antiviral drugs against severe acute respiratory syndrome coronavirus 2 with in silico and in vitro approaches. Biochem. Biophys. Res. Commun. 538, 137–144 (2020).
Guideline on the Clinical Development of Medicinal Products for the Treatment of HIV Infection (European Medicines Agency, 2016); https://go.nature.com/3ZkRSvy
Cho, A. et al. Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg. Med. Chem. Lett. 22, 2705–2707 (2012).
Eastman, R. T. et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 6, 672–683 (2020).
Mulangu, S. et al. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 381, 2293–2303 (2019).
Berliba, E. et al. Safety, pharmacokinetics and antiviral activity of AT-527, a novel purine nucleotide prodrug, in HCV-infected subjects with and without cirrhosis. Antimicrob. Agents Chemother. 63, e01201-19 (2019).
Ueda, M., Tanimoto, T., Murayama, A., Ozaki, A. & Kami, M. Japan’s drug regulation during the COVID-19 pandemic: lessons from a case study of favipiravir. Clin. Pharmacol. Ther. 111, 545–547 (2022).
Popowska, E. & Janion, C. N4-hydroxycytidine-a new mutagen of a base analogue type. Biochem. Biophys. Res. Commun. 56, 459–466 (1974).
Salganik, R. I., Vasjunina, E. A., Poslovina, A. S. & Andreeva, I. S. Mutagenic action of N4-hydroxycytidine on Escherichia coli B cyt. Mutat. Res 20, 1–5 (1973).
Sledziewska, E. & Janion, C. Mutagenic specificity of N4-hydroxycytidine. Mutat. Res. 70, 11–16 (1980).
Toots, M. & Plemper, R. K. Next-generation direct-acting influenza therapeutics. Transl. Res. 220, 33–42 (2020).
Toots, M. et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 11, eaax5866 (2019).
Yoon, J. J. et al. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob. Agents Chemother. 62, e00766-18 (2018).
Sheahan, T. P. et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 12, eabb5883 (2020).
The Little Pill that Could: Emory’s George Painter Led the Discovery of Monupiravir, a Key Tool to Fight COVID-19 (Emory University, 19 November 2021); https://go.nature.com/3ztowRv
ALINIA Prescribing Information (Romark, 2016); https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021497s001,021498s004lbl.pdf
Lokhande, A. S. & Devarajan, P. V. A review on possible mechanistic insights of nitazoxanide for repurposing in COVID-19. Eur. J. Pharmacol. 891, 173748 (2021).
Caly, L., Druce, J. D., Catton, M. G., Jans, D. A. & Wagstaff, K. M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 178, 104787 (2020).
Lehrer, S. & Rheinstein, P. H. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. In Vivo 34, 3023–3026 (2020).
Abd-Elsalam, S. et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study. J. Med. Virol. 93, 5833–5838 (2021).
Reis, G. et al. Effect of early treatment with ivermectin among patients with Covid-19. N. Engl. J. Med. 386, 1721–1731 (2022).
FDA Approves First Treatment for COVID-19 (US FDA, 22 October 2020); https://go.nature.com/3K8REn5
Beigel, J. H. et al. Remdesivir for the treatment of Covid-19—final report. N. Engl. J. Med. 383, 1813–1826 (2020).
Ader, F. et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 22, 209–221 (2021).
Mehta, R. M., Bansal, S., Bysani, S. & Kalpakam, H. A shorter symptom onset to remdesivir treatment (SORT) interval is associated with a lower mortality in moderate-to-severe COVID-19: a real-world analysis. Int. J. Infect. Dis. 106, 71–77 (2021).
The Lancet Infectious Diseases. Unmet need for COVID-19 therapies in community settings. Lancet Infect. Dis. 21, 1471 (2021).
FDA Takes Actions to Expand Use of Treatment for Outpatients with Mild-to-Moderate COVID-19 (US FDA, 21 January 2022); https://go.nature.com/3nlxtt8
Vermillion, M. S. et al. Inhaled remdesivir reduces viral burden in a nonhuman primate model of SARS-CoV-2 infection. Sci. Transl. Med. 14, eabl8282 (2022).
Cox, R. M. et al. Oral prodrug of remdesivir parent GS-441524 is efficacious against SARS-CoV-2 in ferrets. Nat. Commun. 12, 6415 (2021).
Cao, Z. et al. VV116 versus nirmatrelvir–ritonavir for oral treatment of COVID-19. N. Engl. J. Med. 388, 406–417 (2023).
Junshi Biosciences Announces Approval for Marketing of VV116 in China. Financial Post https://go.nature.com/3Kbh17C (30 January 2023).
Atea Pharmaceuticals Provides Update and Topline Results for Phase 2 MOONSONG Trial Evaluating AT-527 in the Outpatient Setting (Atea Pharmaceuticals, 19 October 2021); https://go.nature.com/3nn6tto
Agrawal, U., Raju, R. & Udwadia, Z. F. Favipiravir: a new and emerging antiviral option in COVID-19. Med. J. Armed Forces India 76, 370–376 (2020).
Bosaeed, M. et al. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin. Microbiol. Infect. 28, 602–608 (2022).
Rocco, P. R. M. et al. Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial. Eur. Respir. J. 58, 2003725 (2021).
Nitazoxanide (NIH, accessed 20 November 2022); https://go.nature.com/3U2gUil
Bramante, C. T. et al. Randomized trial of metformin, ivermectin, and fluvoxamine for COVID-19. N. Engl. J. Med. 387, 599–610 (2022).
Halford, B. An emerging antiviral takes aim at COVID-19. Chemical and Engineering News (5 May 2020).
Painter, W. P. et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob. Agents Chemother. 65, e02428-20 (2021).
Riva, L. et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 586, 113–119 (2020).
Singh, A. K., Singh, A., Singh, R. & Misra, A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab. Syndr. 15, 102329 (2021).
Merck and Ridgeback Biotherapeutics Provide Update on Progress of Clinical Development Program for Molnupiravir, an Investigational Oral Therapeutic for the Treatment of Mild-to-Moderate COVID-19 (MSD, 15 April 2021); https://go.nature.com/3JMvl5a
Butler, C. C. et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 401, 281–293 (2023).
Fountain-Jones, N.M. et al. Antiviral treatments lead to the rapid accrual of hundreds of SARS-CoV-2 mutations in 2 immunocompromised patients. Preprint at medRxiv https://doi.org/10.1101/2022.12.21.22283811 (2022).
Bill & Melinda Gates Foundation, Wellcome, and Mastercard Launch Initiative to Speed Development and Access to Therapies for COVID-19 (Bill & Melinda Gates Foundation, 10 March 2020); https://go.nature.com/40GsIIP
Bakowski, M. A. et al. Drug repurposing screens identify chemical entities for the development of COVID-19 interventions. Nat. Commun. 12, 3309 (2021).
Billich, A. Drug evaluation: apilimod, an oral IL-12/IL-23 inhibitor for the treatment of autoimmune diseases and common variable immunodeficiency. IDrugs 10, 53–59 (2007).
Tkach Tuzman, K. Verge puts ALS target through COVID-19 paces, leaning on preclinical partners. Biocentury https://www.biocentury.com/article/305692/verge-puts-als-target-through-covid-19-paces-leaning-on-preclinical-partners (2020).
Talukdar, R., Saikia, N., Singal, D. K. & Tandon, R. Chronic pancreatitis: evolving paradigms. Pancreatology 6, 440–449 (2006).
Chupp, G. et al. A phase 2 randomized, double-blind, placebo-controlled trial of oral camostat mesylate for early treatment of COVID-19 outpatients showed shorter illness course and attenuation of loss of smell and taste. Preprint at medRxiv. https://doi.org/10.1101/2022.01.28.2227003 (2022).
Gunst, J. D. et al. Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19—a double-blind randomized controlled trial. eClinicalMedicine 35, 100849 (2021).
Camostat Efficacy vs. Placebo for Outpatient Treatment of COVID-19 (CAMELOT) (ClinicalTrials.gov, accessed 31 October 2022); https://go.nature.com/40GXrFE
CAMOVID: Evaluation of Efficacy and Safety of Camostat Mesylate for the Treatment of SARS-CoV-2 Infection—COVID-19 in Ambulatory Adult Patients (CAMOVID) (ClinicalTrials.gov, accessed 31 October 2022); https://clinicaltrials.gov/ct2/show/NCT04608266
Oral Camostat Compared with Standard Supportive Care in Mild-Moderate COVID-19 Patients (COPS-2003) (ClinicalTrials.gov, accessed 31 October 2022); https://www.clinicaltrials.gov/ct2/show/NCT04524663
Focosi, D., Anderson, A. O., Tang, J. W. & Tuccori, M. Convalescent plasma therapy for COVID-19: state of the art. Clin. Microbiol. Rev. 33, e00072-20 (2020).
Corti, D. et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 351, 1339–1342 (2016).
Misasi, J. et al. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science 351, 1343–1346 (2016).
An EUA for casirivimab and imdevimab for COVID-19. Med. Lett. Drugs Ther. 62, 201–202 (2020).
An EUA for bamlanivimab and etesevimab for COVID-19. Med. Lett. Drugs Ther. 63, 49–50 (2021).
Fact Sheet for Healthcare Providers: Emergency Use Authorization for Sotrovimab (GSK, 2023); https://go.nature.com/3LTnEg6
Coronavirus (COVID-19) Update: FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 that Retains Activity Against Omicron Variant (US FDA, 11 February 2022); https://go.nature.com/3ZkWIJe
Coronavirus (COVID-19) Update: FDA Authorizes New Long-Acting Monoclonal Antibodies for Pre-exposure Prevention of COVID-19 in Certain Individuals (US FDA, 8 December 2021); https://go.nature.com/40C7Mmv
Recovery Collaborative Group. et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 384, 693–704 (2021).
An EUA for baricitinib (Olumiant) for COVID-19. Med Lett Drugs Ther 62, 202–203 (2020).
Coronavirus (COVID-19) Update: FDA Authorizes Drug Combination for Treatment of COVID-19 (US FDA, 19 November 2020); https://go.nature.com/3K9q4WG
Lamontagne, F. et al. A living WHO guideline on drugs for COVID-19. Brit. Med. J. 370, m3379 (2020).
WHO Recommends Two New Drugs to Treat COVID-19 (WHO, 2022); https://go.nature.com/42IHUqQ
COVID-19 update: baricitinib (Olumiant) FDA-approved for treatment of COVID-19. Med. Lett. Drugs Ther. 64, e2–e3 (2022).
FDA Approves Lilly and Incyte’s OLUMIANT (Baricitinib) for the Treatment of Certain Hospitalized Patients with COVID-19 (Eli Lilly and Company, 11 May 2022); https://go.nature.com/3LV87wC
Withdrawal of Application to Change the Marketing Authorisation for Olumiant (Baricitinib) (EMA, 2022).
Cavalli, G. et al. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. Lancet Rheumatol. 3, e253–e261 (2021).
Recovery Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397, 1637–1645 (2021).
Coronavirus (COVID-19) Update: FDA Authorizes Drug for Treatment of COVID-19 (US FDA, 24 June 2021); https://go.nature.com/3nta9JR
Hemann, E. A., Gale, M. Jr. & Savan, R. Interferon lambda genetics and biology in regulation of viral control. Front. Immunol. 8, 1707 (2017).
Andersen, H. et al. Peginterferon lambda-1a, a new therapeutic for hepatitis C infection, from bench to clinic. J. Clin. Transl. Hepatol. 1, 116–124 (2013).
Eiger BioPharmaceuticals. Eiger’s single-dose peginterferon lambda for COVID-19 reduced risk of hospitalization or ER visits by 50% in a predominantly vaccinated population in phase 3 TOGETHER study. Cision PR Newswire https://go.nature.com/3LVaO16 (17 May 2022).
Barnette, K.G., et. al. Oral sabizabulin for high-risk, hospitalized adults with COVID-19: interim analysis. NEJM Evidence https://doi.org/10.1056/EVIDoa2200145 (2022).
Sullivan, M. G. Veru’s stops COVID-19 treatment trial in face of ‘overwhelmingly positive’ results. FDA News (13 April 2022); https://go.nature.com/3FVWMbP
Zhu, W., Shyr, Z., Lo, D. C. & Zheng, W. Viral proteases as targets for Coronavirus Disease 2019 drug development. J. Pharmacol. Exp. Ther. 378, 166–172 (2021).
de Leuw, P. & Stephan, C. Protease inhibitors for the treatment of hepatitis C virus infection. GMS Infect. Dis. 5, Doc08 (2017).
Voshavar, C. Protease inhibitors for the treatment of HIV/AIDS: recent advances and future challenges. Curr. Top. Med. Chem. 19, 1571–1598 (2019).
Hoffman, R. et al. The discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J. Med. Chem. 63, 12725–12747 (2020).
Boras, B. et al. Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19. Nat. Commun. 12, 6055 (2021).
Pfizer Reports Fourth-Quarter and Full-Year 2021 Results (Pfizer, 2022); https://go.nature.com/40ITyjT
Owen, D. R. et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 374, 1586–1593 (2021).
Eng, H. et al. Disposition of nirmatrelvir, an orally bioavailable inhibitor of SARS-CoV-2 3C-like protease, across animals and humans. Drug Metab. Dispos. 50, 576–590 (2022).
Pfizer Shares Top-Line Results from Phase 2/3 EPIC-PEP Study of PAXLOVID for Post-Exposure Prophylactic Use (Pfizer, 29 April 2022); https://go.nature.com/3JGKdSs
Pfizer Reports Additional Data on PAXLOVID Supporting Upcoming New Drug Application Submission to U.S. FDA (Pfizer, 14 June 2022); https://go.nature.com/42DnbEZ
Greasley, S. E. et al. Structural basis for the in vitro efficacy of nirmatrelvir against SARS-CoV-2 variants. J. Biol. Chem. 298, 101972 (2022).
Rai, D. K. et al. Nirmatrelvir, an orally active Mpro inhibitor, is a potent inhibitor of SARS-CoV-2 Variants of Concern. Preprint at bioRxiv https://doi.org/10.1101/2022.01.17.476644 (2022).
Rosales, R. et al. Nirmatrelvir, molnupiravir, and remdesivir maintain potent in vitro activity against the SARS-CoV-2 Omicron variant. Preprint at bioRxiv https://doi.org/10.1101/2022.01.17.476685 (2022).
Vangeel, L. et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivir. Res. 198, 105252 (2022).
Li, P. et al. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 32, 322–324 (2022).
Lee, J. T. et al. Genetic surveillance of SARS-CoV-2 Mpro reveals high sequence and structural conservation prior to the introduction of protease inhibitor Paxlovid. mBio 13, e0086922 (2022).
Iketani, S. et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature 613, 558–564 (2023).
Jochmans, D. et al. The substitutions L50F, E166A and L167F in SARS-CoV-2 3CLpro are selected by a protease inhibitor in vitro and confer resistance to nirmatrelvir. mBio 14, e0281522 (2023).
Zhou, Y. et al. Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system. Sci. Adv. 8, eadd7197 (2022).
Fact Sheet for Healthcare Providers: Emergency Use Authorization for PAXLOVID (Pfizer, 2023); https://go.nature.com/3zbxvqg
NORVIR Prescribing Information (AbbVie, 2017); https://go.nature.com/3JPjxiB
Management of Drug Interactions with Nirmatrelvir/Ritonavir (Paxlovid): Resource for Clinicians (IDSA, 6 May 2022); https://go.nature.com/3JKJqQJ
Notice Regarding the Initiation of a Phase 1 Clinical Trial for a COVID-19 Therapeutic Agent in Japan (Shionogi, 26 July 2021); https://go.nature.com/3LUqS2Y
Unoh, Y. et al. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J. Med. Chem. 65, 6499–6512 (2022).
Notice Regarding the Initiation of a Phase 2/3 Clinical Trial for a COVID-19 Therapeutic Agent in Japan (Shionogi, 28 September 2021); https://go.nature.com/42LsS3O
New Data for Shionogi’s COVID-19 Once-Daily Oral Antiviral S-217622 Show Rapid Virus Clearance (Shionogi, 23 April 2022); https://go.nature.com/40FJl7O
Todos Medical launches phase 2 clinical trial of its antiviral 3CL protease inhibitor NLC-V-01 (Tollovir) in hospitalized COVID-19 patients. GlobeNewswire (19 April 2021); https://go.nature.com/3nk1o4R
Todos Medical to present final data from the Tollovir Phase 2 clinical trial in hospitalized COVID-19 patients at the Personalized Medicine World Conference. Bloomberg (27 May 2022); https://go.nature.com/3zb0t9F
Todos Medical and NLC Pharma Announce Primary and Secondary Endpoints Met in NLC-V-01 Phase 2 Clinical Trial of Oral Antiviral 3CL Protease Inhibitor Tollovir in the Treatment of Hospitalized COVID-19 Patients (Todos Medical Ltd., 27 January 2022); https://go.nature.com/3Zk0brF
Pomotrelvir, an Investigational Coronavirus Main Protease (Mpro) Inhibitor (Pardes Biosciences); https://go.nature.com/3nrxJXo
Pardes Biosciences announces commencement of phase 2 trial evaluating PBI-0451 for the treatment of SARS-CoV-2 infections. Bloomberg https://go.nature.com/3TMLGLI (13 September 2022).
Enanta Pharmaceuticals announces positive data from a phase 1 clinical study of EDP-235, its oral 3CL protease inhibitor designed for the treatment of COVID-19. Business Wire https://go.nature.com/3LYrYL9 (29 July 2022).
Aligos Therapeutics Selects Drug Candidate ALG-097558, a Potent Ritonavir-Free Oral Protease Inhibitor for the Treatment and Prevention of COVID-19, to Advance into Development (Aligos Therapeutics, 4 April 2022); https://go.nature.com/3Zmwje2
Stumpp, M. T., Dawson, K. M. & Binz, H. K. Beyond antibodies: The DARPin drug platform. BioDrugs 34, 423–433 (2020).
Walser, M. et al. Highly potent anti-SARS-CoV-2 multivalent DARPin therapeutic candidates. Preprint at bioRxiv https://doi.org/10.1101/2020.08.25.256339v3 (2021).
Rothenberger, S. et al. The trispecific DARPin ensovibep inhibits diverse SARS-CoV-2 variants. Nat. Biotechnol. 40, 1845–1854 (2022).
Novartis and Molecular Partners Report Positive Topline Data from Phase 2 Study for Ensovibep (MP0420), a DARPin Antiviral Therapeutic for COVID-19 (Novartis, 10 January 2022); https://go.nature.com/3U2nDc5
Jia, H., Neptune, E. & Cui, H. Targeting ACE2 for COVID-19 therapy: opportunities and challenges. Am. J. Respir. Cell Mol. Biol. 64, 416–425 (2021).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280 (2020).
Rahbar Saadat, Y., Hosseiniyan Khatibi, S. M., Zununi Vahed, S. & Ardalan, M. Host serine proteases: a potential targeted therapy for COVID-19 and influenza. Front. Mol. Biosci. 8, 725528 (2021).
Shang, J. et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl Acad. Sci. USA 117, 11727–11734 (2020).
Doi, K. et al. Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with COVID-19: a case series. Crit. Care 24, 392 (2020).
Hofmann-Winkler, H. et al. Camostat mesylate may reduce severity of Coronavirus Disease 2019 sepsis: a first observation. Crit. Care Explor. 2, e0284 (2020).
Quinn, T. M. et al. Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: phase 1b/2a experimental study to investigate safety, pharmacokinetics and pharmacodynamics. eBioMedicine 76, 103856 (2022).
Richardson, P. et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395, e30–e31 (2020).
Assadiasl, S., Fatahi, Y., Mosharmovahed, B., Mohebbi, B. & Nicknam, M. H. Baricitinib: from rheumatoid arthritis to COVID-19. J. Clin. Pharm. 61, 1274–1285 (2021).
Chodera, J., Lee, A. A., London, N. & von Delft, F. Crowdsourcing drug discovery for pandemics. Nat. Chem. 12, 581 (2020).
Shin, D. et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 587, 657–662 (2020).
Cheng, V. C., Lau, S. K., Woo, P. C. & Yuen, K. Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 20, 660–694 (2007).
Ball, P. The lightning-fast quest for COVID vaccines—and what it means for other diseases. Nature 589, 16–18 (2021).
Tartof, S. Y. et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the Omicron and Delta variants in a large health system in the USA: a test-negative case-control study. Lancet Respir. Med. 10, 689–699 (2022).
Tenforde, M. W. et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing COVID-19 hospitalizations in the United States. Clin. Infect. Dis. 74, 1515–1524 (2021).
Strategic Preparedness, Readiness and Response Plan to End the Global COVID-19 Emergency in 2022 (WHO, 2022); https://go.nature.com/3M0eSNH
Buskermolen, M. et al. Relapse in the first 8 weeks after onset of COVID-19 disease in outpatients: viral reactivation or inflammatory rebound? J. Infect. 83, e6–e8 (2021).
Gousseff, M. et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J. Infect. 81, 816–846 (2020).
Wang, L. et al. COVID-19 rebound after Paxlovid and Molnupiravir during January–June 2022. Preprint at medRxiv https://doi.org/10.1101/2022.06.21.22276724 (2022).
Anderson, A. S., Caubel, P. & Rusnak, J. M. & EPIC-HR Trial Investigators. Nirmatrelvir–ritonavir and viral load rebound in COVID-19. N. Engl. J. Med. 387, 1047–1049 (2022).
Charness, M. E. et al. Rebound of SARS-CoV-2 infection after nirmatrelvir–ritonavir treatment. N. Engl. J. Med. 387, 1045–1047 (2022).
Chen, C. et al. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J. Infect. Dis. 226, 1593–1607 (2022).
Chang, S. E. et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 12, 5417 (2021).
Sapkota, H. R. & Nune, A. Long COVID from rheumatology perspective—a narrative review. Clin. Rheumatol. 41, 337–348 (2022).
Su, Y. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185, 881–895 (2022).
Brito-Zeron, P. et al. Post-COVID-19 syndrome in patients with primary Sjogren’s syndrome after acute SARS-CoV-2 infection. Clin. Exp. Rheumatol. 39, 57–65 (2021).
COVID-19 Vaccinations in the United States (Centers for Disease Control and Prevention, accessed 15 February 2023); https://go.nature.com/3TKuk29
Johnson, A. G. et al. COVID-19 incidence and mortality among unvaccinated and vaccinated persons aged ≥12 years by receipt of bivalent booster doses and time since vaccination — 24 U.S. jurisdictions, October 3, 2021–December 24, 2022. MMWR Morb. Mortal. Wkly. Rep. 72, 145–152 (2023).
Kocks, J. et al. A potential harmful effect of dexamethasone in non-severe COVID-19: results from the COPPER-pilot study. ERJ Open Res. 8, 00129–02022 (2022).
Shuto, H. et al. A systematic review of corticosteroid treatment for noncritically ill patients with COVID-19. Sci. Rep. 10, 20935 (2020).
Corticosteroids (NIH, 31 May 2022); https://go.nature.com/3U2oFot
Coronavirus (COVID-19) Update: FDA Approves First COVID-19 Treatment for Young Children (US FDA, 25 April 2022); https://go.nature.com/3ZjtVVA
Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19 (US FDA, 22 December 2021); https://go.nature.com/3ZkYMBb
Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults (US FDA, 23 December 2021); https://go.nature.com/3Zj1yXI
Cavazzoni, P. Coronavirus (COVID-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat COVID-19 Due to the Omicron Variant (US FDA, 24 January 2022); https://go.nature.com/3FW0J09
FDA Expands Authorization of Two Monoclonal Antibodies for Treatment and Post-Exposure Prevention of COVID-19 to Younger Pediatric Patients, Including Newborns (US FDA, 3 December 2021); https://go.nature.com/40D8z6z
An EUA for sotrovimab for treatment of COVID-19. Med. Lett. Drugs Ther. 63, 97–98 (2021).
FDA Updates Sotrovimab Emergency Use Authorization (US FDA, 5 April 2022); https://go.nature.com/3TPmZhZ
FDA Announces Bebtelovimab is not Currently Authorized in Any US Region (US FDA, 30 November 2022); https://go.nature.com/3lIdBA1
An EUA for bebtelovimab for treatment of COVID-19. Med. Lett. Drugs Ther. 64, 41–42 (2022).
FDA announces Evusheld is not Currently Authorized for Emergency Use in the U.S. (US FDA, 26 December 2022); https://go.nature.com/3TPeh33
Emergency Use Authorization: Drugs and Non-Vaccine Biological Products (US FDA, accessed on 3 February 2023); https://go.nature.com/3K86kml
Therapeutic Management of Nonhospitalized Children With COVID-19 (NIH, 2022); https://go.nature.com/3LVck37
Therapeutic Management of Hospitalized Children with COVID-19 (NIH, 8 August 2022); https://go.nature.com/3LRRGRB
Chen, Y., Liu, Q. & Guo, D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 92, 418–423 (2020).
Dos Santos, W. G. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed. Pharmacother. 129, 110493 (2020).
Moore, J. B. & June, C. H. Cytokine release syndrome in severe COVID-19. Science 368, 473–474 (2020).
Ye, Q., Wang, B. & Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 80, 607–613 (2020).
Konstantinidou, S. K. & Papanastasiou, I. P. Repurposing current therapeutic regimens against SARS-CoV-2 (Review). Exp. Ther. Med. 20, 1845–1855 (2020).
Farooqi, F. et al. Treatment of severe COVID-19 with tocilizumab mitigates cytokine storm and averts mechanical ventilation during acute respiratory distress: a case report and literature review. Trop. Med. Infect. Dis. 5, 112 (2020).
Antivirals (IDSA, 2022); https://go.nature.com/40ik7fH
Kabinger, F. et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 28, 740–746 (2021).
Fact Sheet for Healthcare Providers: Emergency Use Authorization for Lagevrio (Molnupiravir) Capsules (MDS, 2023); https://go.nature.com/42Krjmp
Acknowledgements
We thank the following of our colleagues at Pfizer: C. Allerton for critical reading of the manuscript, N. Kushnir for editorial assistance and A. Merrifield for assistance in graphics preparation.
Author information
Authors and Affiliations
Contributions
S.S.T., J.L.H., B.S.G. and A.S.A. wrote the manuscript, and read and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors are paid employees of Pfizer Inc. and own Pfizer stock at the time of writing this manuscript.
Peer review
Peer review information
Nature Microbiology thanks Koichi Watashi, Wagner Dos Santos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toussi, S.S., Hammond, J.L., Gerstenberger, B.S. et al. Therapeutics for COVID-19. Nat Microbiol 8, 771–786 (2023). https://doi.org/10.1038/s41564-023-01356-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-023-01356-4