Abstract
Apicomplexan parasites secrete contents of the rhoptries, club-shaped organelles in the apical region, into host cells to permit their invasion and establishment of infection. The rhoptry secretory apparatus (RSA), which is critical for rhoptry secretion, was recently discovered in Toxoplasma and Cryptosporidium. It is unknown whether a similar molecular machinery exists in the malaria parasite Plasmodium. In this study, we use in situ cryo-electron tomography to investigate the rhoptry secretion system in P. falciparum merozoites. We identify the presence of an RSA at the cell apex and a morphologically distinct apical vesicle docking the tips of the two rhoptries to the RSA. We also discover two additional rhoptry organizations that lack the apical vesicle. Using subtomogram averaging, we reveal different conformations of the RSA structure corresponding to different rhoptry organizations. Our results highlight previously unknown steps in the process of rhoptry secretion and indicate a regulatory role for the conserved apical vesicle in host invasion by apicomplexan parasites.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Representative tomograms showing different morphological states of P. falciparum rhoptry fusion (Fig. 2a,b,d) are available in the Electron Microscopy Data Bank (EMDB) under accession codes EMD-26745 (Fig. 2a), EMD-26746 (Fig. 2b) and EMD-26747 (Fig. 2d). Subtomogram averages of the P. falciparum RSA in different conformations are available in the EMDB under accession codes EMD-26670 (RSA-A), EMD-26671 (RSA-2R) and EMD-26672 (RSA-1R). Source data are provided with this paper.
References
Cowman, A. F., Healer, J., Marapana, D. & Marsh, K. Malaria: biology and disease. Cell 167, 610–624 (2016).
Blader, I. J., Coleman, B. I., Chen, C.-T. & Gubbels, M.-J. Lytic cycle of Toxoplasma gondii: 15 years later. Annu. Rev. Microbiol 69, 463–485 (2015).
Guérin, A. & Striepen, B. The biology of the intestinal intracellular parasite Cryptosporidium. Cell Host Microbe 28, 509–515 (2020).
Dubois, D. J. & Soldati‐Favre, D. Biogenesis and secretion of micronemes in Toxoplasma gondii. Cell. Microbiol. 21, e13018 (2019).
Ben Chaabene, R., Lentini, G. & Soldati‐Favre, D. Biogenesis and discharge of the rhoptries: key organelles for entry and hijack of host cells by the Apicomplexa. Mol. Microbiol. https://doi.org/10.1111/mmi.14674 (2020).
Riglar, D. T. et al. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe 9, 9–20 (2011).
Sparvoli, D. & Lebrun, M. Unraveling the elusive rhoptry exocytic mechanism of Apicomplexa. Trends Parasitol. https://doi.org/10.1016/j.pt.2021.04.011 (2021).
Aquilini, E. et al. An Alveolata secretory machinery adapted to parasite host cell invasion. Nat. Microbiol. https://doi.org/10.1038/s41564-020-00854-z (2021).
Mageswaran, S. K. et al. In situ ultrastructures of two evolutionarily distant apicomplexan rhoptry secretion systems. Nat. Commun. 12, 4983 (2021).
Hanssen, E. et al. Electron tomography of Plasmodium falciparum merozoites reveals core cellular events that underpin erythrocyte invasion. Cell. Microbiol. 15, 1457–1472 (2013).
Aikawa, M., Miller, L. H., Johnson, J. & Rabbege, J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J. Cell Biol. 77, 72–82 (1978).
Singh, S., Alam, M. M., Pal-Bhowmick, I., Brzostowski, J. A. & Chitnis, C. E. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 6, e1000746 (2010).
Lepper, S., Merkel, M., Sartori, A., Cyrklaff, M. & Frischknecht, F. Rapid quantification of the effects of blotting for correlation of light and cryo-light microscopy images. J. Microsc. 238, 21–26 (2010).
Kudryashev, M. et al. Structural basis for chirality and directional motility of Plasmodium sporozoites. Cell. Microbiol. 14, 1757–1768 (2012).
Cyrklaff, M., Frischknecht, F. & Kudryashev, M. Functional insights into pathogen biology from 3D electron microscopy. FEMS Microbiol. Rev. 41, 828–853 (2017).
Theveny, L. M. et al. Parasitology meets cryo-electron tomography – exciting prospects await. Trends Parasitol. https://doi.org/10.1016/J.PT.2022.01.006 (2022).
Miller, L. H., Aikawa, M., Johnson, J. G. & Shiroishi, T. Interaction between cytochalasin B-treated malarial parasites and erythrocytes. J. Exp. Med. 149, 172–184 (1979).
Cowman, A. F., Tonkin, C. J., Tham, W.-H. & Duraisingh, M. T. The molecular basis of erythrocyte invasion by malaria parasites. Cell Host Microbe https://doi.org/10.1016/j.chom.2017.07.003 (2017).
Liffner, B. et al. PfCERLI1 is a conserved rhoptry associated protein essential for Plasmodium falciparum merozoite invasion of erythrocytes. Nat. Commun. 11, 1411 (2020).
Sherling, E. S. et al. The Plasmodium falciparum rhoptry bulb protein RAMA plays an essential role in rhoptry neck morphogenesis and host red blood cell invasion. PLoS Pathog. 15, e1008049 (2019).
Rudlaff, R. M., Kraemer, S., Marshman, J. & Dvorin, J. D. Three-dimensional ultrastructure of Plasmodium falciparum throughout cytokinesis. PLoS Pathog. 16, e1008587 (2020).
Tetley, L., Brown, S. M. A., Mcdonald, V. & Coombsl, G. H. Ultrastructural analysis of the sporozoite of Cryptosporidium parvum. Microbiology 144, 3249–3255 (1998).
Paredes-Santos, T. C., De Souza, W. & Attias, M. Dynamics and 3D organization of secretory organelles of Toxoplasma gondii. J. Struct. Biol. 177, 420–430 (2012).
Dubremetz, J. F. Rhoptries are major players in Toxoplasma gondii invasion and host cell interaction. Cell. Microbiol. 9, 841–848 (2007).
Bisson, C., Hecksel, C. W., Gilchrist, J. B. & Fleck, R. A. Preparing lamellae from vitreous biological samples using a dual-beam scanning electron microscope for cryo-electron tomography. J. Vis. Exp. https://doi.org/10.3791/62350 (2021).
Burrell, A. et al. Cellular electron tomography of the apical complex in the apicomplexan parasite Eimeria tenella shows a highly organised gateway for regulated secretion. Preprint at bioRxiv https://doi.org/10.1101/2021.06.17.448283 (2021).
Plattner, H. Trichocysts–Paramecium’s projectile-like secretory organelles: reappraisal of their biogenesis, composition, intracellular transport, and possible functions. J. Eukaryot. Microbiol. 64, 106–133 (2017).
Coleman, B. I. et al. A member of the ferlin calcium sensor family is essential for Toxoplasma gondii rhoptry secretion. mBio 9, e01510-18 (2018).
Lyth, O. et al. Cellular dissection of malaria parasite invasion of human erythrocytes using viable Plasmodium knowlesi merozoites. Sci. Rep. 8, 10165 (2018).
Aikawa, M., Miller, L. H., Rabbege, J. R. & Epstein, N. Freeze-fracture study on the erythrocyte membrane during malarial parasite invasion. J. Cell Biol. 91, 55–62 (1981).
Liffner, B., Balbin, J. M., Wichers, J. S., Gilberger, T.-W. & Wilson, D. W. The ins and outs of Plasmodium rhoptries, focusing on the cytosolic side. Trends Parasitol. https://doi.org/10.1016/J.PT.2021.03.006 (2021).
Nichols, B. A., Chiappino, M. L. & Richard O’connor, G. Secretion from the rhoptries of Toxoplasma gondii during host-cell invasion. J. Ultrastruct. Res. 83, 85–98 (1983).
Baum, J. et al. Reticulocyte-binding protein homologue 5 – an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int. J. Parasitol. 39, 371–380 (2009).
Counihan, N. A., Kalanon, M., Coppel, R. L. & De Koning-Ward, T. F. Plasmodium rhoptry proteins: why order is important. Trends Parasitol. 29, 228–236 (2013).
de Oliveira, L. S. et al. Calcium in the backstage of malaria parasite biology. Front. Cell. Infect. Microbiol. 11, 708834 (2021).
Volz, J. C. et al. Essential role of the PfRh5/PfRipr/CyRPA complex during Plasmodium falciparum invasion of erythrocytes. Cell Host Microbe 20, 60–71 (2016).
Weiss, G. E. et al. Revealing the sequence and resulting cellular morphology of receptor-ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog. 11, e1004670 (2015).
Suarez, C. et al. A lipid-binding protein mediates rhoptry discharge and invasion in Plasmodium falciparum and Toxoplasma gondii parasites. Nat. Commun. 10, 4041 (2019).
Boyle, M. J. et al. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc. Natl Acad. Sci. USA 107, 14378–14383 (2010).
Iancu, C. V. et al. Electron cryotomography sample preparation using the Vitrobot. Nat. Protoc. 1, 2813–2819 (2006).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Xuong, N. H. et al. Future directions for camera systems in electron microscopy. Methods Cell. Biol. 79, 721–739 (2007).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Danev, R., Buijsse, B., Khoshouei, M., Plitzko, J. M. & Baumeister, W. Volta potential phase plate for in-focus phase contrast transmission electron microscopy. Proc. Natl Acad. Sci. USA 111, 15635–15640 (2014).
Krivanek, O. L., Friedman, S. L., Gubbens, A. J. & Kraus, B. An imaging filter for biological applications. Ultramicroscopy 59, 267–282 (1995).
Turoňová, B. et al. Benchmarking tomographic acquisition schemes for high-resolution structural biology. Nat. Commun. 11, 876 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Chen, M. et al. Convolutional neural networks for automated annotation of cellular cryo-electron tomograms. Nat. Methods 14, 983–985 (2017).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Castaño-Díez, D., Kudryashev, M., Arheit, M. & Stahlberg, H. Dynamo: a flexible, user-friendly development tool for subtomogram averaging of cryo-EM data in high-performance computing environments. J. Struct. Biol. 178, 139–151 (2012).
Castaño-Díez, D. The Dynamo package for tomography and subtomogram averaging: components for MATLAB, GPU computing and EC2 Amazon Web Services. Acta Cryst. D73, 478–487 (2017).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Acknowledgements
We thank S. Steimle for technical assistance with the Titan Krios G3i cryogenic electron microscope, the Singh Center for Nanotechnology and the Beckman Center for Cryo-Electron Microscopy at the University of Pennsylvania for hosting and supporting the use of the Titan Krios; D. Hodge in the lab of A.R.O.J. for training in P. falciparum culture techniques; L. Theveny from the lab of Y.-W.C. for providing subtomogram averages of the T. gondii and C. parvum RSAs; and other members of the labs of Y.-W.C., A.R.O.J. and M.L. for overall support and useful discussions. This work was supported in part by a David and Lucile Packard Fellowship for Science and Engineering (2019–69645) and a Pennsylvania Department of Health FY19 Health Research Formula Fund to Y.-W.C.; a Martin and Pamela Winter Infectious Disease Fellowship to M.M.; the Mary L. and Matthew S. Santirocco College Alumni Society Undergraduate Research Grant to W.D.C.; an EMBO fellowship (ALTF 58–2018) to A.G.; NIH/NIAID R01 AI103280, R21 AI123808, R21 AI130584 and R61 DH105594 to A.R.O.J. who is an Investigator in the Pathogenesis of Infectious Diseases (PATH) of the Burroughs Wellcome Fund; and a European Research Council advanced grant 833309 (KissAndSpitRhoptry) to M.L.
Author information
Authors and Affiliations
Contributions
M.M., M.L. and Y.-W.C. conceptualized and designed the experiments. M.M. cultured and isolated parasites provided by A.R.O.J. M.M.C. provided a protocol and consultations for the efficient isolation of merozoites. P.M. provided further training and useful insights for P. falciparum culture and merozoite isolation. M.M. prepared frozen grids and performed cryo-ET, with training from S.K.M., using an automated data-processing pipeline for on-the-fly tomogram reconstruction that was established by W.D.C., who also provided additional computational support during data collection, processing and management. M.M. analysed the tomograms, performed subtomogram averaging and analysed the RSA structure. W.D.C. performed data analysis of rhoptry volumes. S.K.M., A.G., M.M.C. and M.L. provided important insights for the interpretation of data. M.M. prepared the manuscript, with critical inputs and revisions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Peer review
Peer review information
Nature Microbiology thanks Friedrich Frischknecht, Ke Hu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
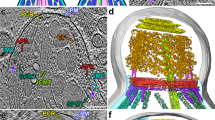
Extended Data Fig. 1 The apical complex and apical vesicle of P. falciparum merozoites.
(a) Simplified schematic of a P. falciparum merozoite apical region. (b–d) 2-D slices through a tomogram of a top-down view of the merozoite apical complex with color overlays of all the observed apical end components: apical vesicle (AV; pink), parasite plasma membrane (PPM; blue), rhoptry (orange), rhoptry secretory apparatus (RSA; light blue), microneme (yellow), inner membrane complex (IMC; green), preconoidal rings (brown), apical polar ring (purple), and subpellicular microtubules (cyan). The cross-sectional planes used in b-d are arranged such that they start at the parasite apex and move inwards into the cell. Scale bar = 100 nm. (e, f) Additional examples of 2-D slices of the AV and rhoptry (left). Blue and orange lines across the AV and rhoptry, respectively, denote the locations of pixel values plotted on the right. Arrows in the pixel plots point to peaks in values corresponding to membranes. Scale bars = 50 nm.
Extended Data Fig. 2 2-D slices through the P. falciparum merozoite apical complex.
(a–c) 2-D slices from tomograms showing either one rhoptry (a, b) or no rhoptries (c) docked at the AV. Scale bars = 50 nm. (d) 2-D slice from a P. falciparum merozoite apical end tomogram within a mature schizont, adapted from [30]. (e) The same 2-D slice from panel j with the original color overlay of the rhoptry of interest, according to [30]. (f) The same 2-D slice from panel j with an updated color overlay of the rhoptry of interest, the AV, and the RSA, based on our analysis. Scale bars = 200 nm.
Extended Data Fig. 3 Comparison of the apical vesicles from P. falciparum, T. gondii, and C. parvum.
(a) Representative 2-D slices of tomograms displaying the AV from each organism (left – P. falciparum; middle – T. gondii; and right – C. parvum), highlighting the PPM (dark blue), RSA (light blue), AV (pink), and rhoptries (orange). Scale bars = 50 nm. (b) Plots of the shortest distance (AVdist) between the PPM apex and the AV membrane from several cells in each organism, showing the mean ±s.d.. (c) Plots of the major (AVmaj) versus minor axis (AVmin) of the AV from each organism, showing mean ±s.d.. (d) Plots of the angle (Ψ at which the major axis of the AV is oriented with respect to the line connecting the PPM apex and the AV centroid each organism. The median value is shown. N = 39 AVs for P. falciparum, 25 AVs for T. gondii, and 28 AVs for C. parvum.
Extended Data Fig. 4 Various fusion states of the P. falciparum rhoptries.
(a–c) 2-D slices through tomograms of apical ends showing the absence of the apical vesicle at the rhoptry tips and either bulging of one rhoptry towards the other (a, b) or twisting of the rhoptries (c). Dashed orange lines in panel c denote the portion of the rhoptry neck that is out of view. A mesh of electron-dense material (green) can be observed between rhoptries (orange) that display bulging towards the other. One rhoptry is docked directly at the RSA (purple). (d, e) Examples in which the two rhoptries are fused together and docked directly at the RSA. (f, g) Examples in which only one, very large rhoptry with no discernable neck region was observed and docked directly at the RSA. Scale bars = 100 nm.
Extended Data Fig. 5 Subtomogram averages of the P. falciparum RSA.
(a) Schematic of the RSA, apical vesicle, and PPM demonstrating the top view and side view orientations used to generate 2-D slices of the subtomogram averages. (b, c) Side views (b) and top view (c) of the subtomogram average of the RSA-A structure before applying 8-fold symmetry. Left to right panels: from peripheral to central cross sections (b) or from extracellular to intracellular cross sections (c). (d–g) Side views (d, f) and top view (e, g) of the subtomogram average of the RSA-2R structure, before and after applying 8-fold symmetry. (h–k) Side views (h, j) and top views (i, k) of the subtomogram average of the RSA-1R structure, before and after applying 8-fold symmetry. (l) Gold standard Fourier shell correlation plot of the final, 8-fold symmetrized subtomogram averages of the three RSA structures. Scale bars = 10 nm.
Extended Data Fig. 6 Representative cryo-ET images of the P. falciparum AV and RSA.
(a-j) 2-D slices with of merozoite apexes (left panels) with color overlays (right panels) highlighting the RSA (light blue), the AV (pink), and the connections from the RSA A-II/III densities to the AV (encircled in red dashed lines). Scale bars = 50 nm.
Supplementary information
Supplementary Video 1
Three-dimensional view of the subtomogram average of the P. falciparum RSA-A structure.
Supplementary Video 2
Subtomogram average of the P. falciparum RSA-A (left), RSA-2R (middle) and RSA-1R (right) structures slicing first through the top view from extracellular to intracellular and back, followed by slicing through the side views.
Supplementary Video 3
Three-dimensional view of the P. falciparum RSA structure, highlighting conformational changes between the RSA-A and RSA-1R states.
Source data
Source Data Fig. 1
Tables of plotted values for Fig. 1d, e and h–l.
Source Data Fig. 2
Tables of plotted values for Fig. 2e and f.
Source Data Fig. 5
Table of plotted values for Fig. 5e.
Source Data Extended Data Fig. 1
Tables of plotted values for Extended Data Fig. 1e and f.
Source Data Extended Data Fig. 3
Tables of plotted values for Extended Data Fig. 3b–d.
Rights and permissions
About this article
Cite this article
Martinez, M., Chen, W.D., Cova, M.M. et al. Rhoptry secretion system structure and priming in Plasmodium falciparum revealed using in situ cryo-electron tomography. Nat Microbiol 7, 1230–1238 (2022). https://doi.org/10.1038/s41564-022-01171-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-022-01171-3