Abstract
Cyclic-oligonucleotide-based anti-phage signalling systems (CBASS) are a family of defence systems against bacteriophages (hereafter phages) that share ancestry with the cGAS–STING innate immune pathway in animals. CBASS systems are composed of an oligonucleotide cyclase, which generates signalling cyclic oligonucleotides in response to phage infection, and an effector that is activated by the cyclic oligonucleotides and promotes cell death. Cell death occurs before phage replication is completed, therefore preventing the spread of phages to nearby cells. Here, we analysed 38,000 bacterial and archaeal genomes and identified more than 5,000 CBASS systems, which have diverse architectures with multiple signalling molecules, effectors and ancillary genes. We propose a classification system for CBASS that groups systems according to their operon organization, signalling molecules and effector function. Four major CBASS types were identified, sharing at least six effector subtypes that promote cell death by membrane impairment, DNA degradation or other means. We observed evidence of extensive gain and loss of CBASS systems, as well as shuffling of effector genes between systems. We expect that our classification and nomenclature scheme will guide future research in the developing CBASS field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All genomic data that support the findings of this study are available at IMG (https://img.jgi.doe.gov/cgi-bin/mer/main.cgi). Accession codes for all data are provided in Supplementary Tables 1–7. PDB and Pfam databases are available at the HHsuite database page (http://wwwuser.gwdg.de/~compbiol/data/hhsuite/databases/hhsuite_dbs/).
References
Bernheim, A. & Sorek, R. The pan-immune system of bacteria: antiviral defence as a community resource. Nat. Rev. Microbiol. 18, 113–119 (2020).
Doron, S. et al. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359, eaar4120 (2018).
Rostøl, J. T. & Marraffini, L. (Ph)ighting phages: how bacteria resist their parasites. Cell Host Microbe 25, 184–194 (2019).
Hampton, H. G., Watson, B. N. J. & Fineran, P. C. The arms race between bacteria and their phage foes. Nature 577, 327–336 (2020).
Cohen, D. et al. Cyclic GMP–AMP signalling protects bacteria against viral infection. Nature 574, 691–695 (2019).
Davies, B. W., Bogard, R. W., Young, T. S. & Mekalanos, J. J. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 149, 358–370 (2012).
Severin, G. B. et al. Direct activation of a phospholipase by cyclic GMP-AMP in El Tor Vibrio cholerae. Proc. Natl Acad. Sci. USA 115, E6048–E6055 (2018).
Whiteley, A. T. et al. Bacterial cGAS-like enzymes synthesize diverse nucleotide signals. Nature 567, 194–199 (2019).
Ye, Q. et al. HORMA domain proteins and a Trip13-like ATPase regulate bacterial cGAS-like enzymes to mediate bacteriophage immunity. Mol. Cell 77, 709–722 (2020).
Lau, R. K. et al. Structure and mechanism of a cyclic trinucleotide-activated bacterial endonuclease mediating bacteriophage immunity. Mol. Cell 77, 723–733 (2020).
Burroughs, A. M., Zhang, D., Schäffer, D. E., Iyer, L. M. & Aravind, L. Comparative genomic analyses reveal a vast, novel network of nucleotide-centric systems in biological conflicts, immunity and signaling. Nucleic Acids Res. 43, 10633–10654 (2015).
Lowey, B. et al. CBASS immunity uses CARF-related effectors to sense 3′–5′- and 2′–5′-linked cyclic oligonucleotide signals and protect bacteria from phage infection. Cell 182, 38–49.e17 (2020).
Aravind, L. & Koonin, E. V. The HORMA domain: a common structural denominator in mitotic checkpoints, chromosome synapsis and DNA repair. Trends Biochem. Sci. 23, 284–286 (1998).
Rosenberg, S. C. & Corbett, K. D. The multifaceted roles of the HORMA domain in cellular signaling. J. Cell Biol. 211, 745–755 (2015).
Vader, G. Pch2(TRIP13): controlling cell division through regulation of HORMA domains. Chromosoma 124, 333–339 (2015).
Reader, J. S., Metzgar, D., Schimmel, P. & de Crécy-Lagard, V. Identification of four genes necessary for biosynthesis of the modified nucleoside queuosine. J. Biol. Chem. 279, 6280–6285 (2004).
McCarty, R. M., Somogyi, A., Lin, G., Jacobsen, N. E. & Bandarian, V. The deazapurine biosynthetic pathway revealed: in vitro enzymatic synthesis of PreQ(0) from guanosine 5′-triphosphate in four steps. Biochemistry 48, 3847–3852 (2009).
Okada, N. et al. Novel mechanism of post-transcriptional modification of tRNA. Insertion of bases of Q precursors into tRNA by a specific tRNA transglycosylase reaction. J. Biol. Chem. 254, 3067–3073 (1979).
Thiaville, J. J. et al. Novel genomic island modifies DNA with 7-deazaguanine derivatives. Proc. Natl Acad. Sci. USA 113, E1452–E1459 (2016).
David, S. S., O’Shea, V. L. & Kundu, S. Base-excision repair of oxidative DNA damage. Nature 447, 941–950 (2007).
McLennan, A. G. The Nudix hydrolase superfamily. Cell. Mol. Life Sci. 63, 123–143 (2006).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Botsford, J. L. & Harman, J. G. Cyclic AMP in prokaryotes. Microbiol. Rev. 56, 100–122 (1992).
Koonin, E. V., Makarova, K. S. & Wolf, Y. I. Evolutionary genomics of defense systems in archaea and bacteria. Annu. Rev. Microbiol. 71, 233–261 (2017).
van Houte, S., Buckling, A. & Westra, E. R. Evolutionary ecology of prokaryotic immune mechanisms. Microbiol. Mol. Biol. Rev. 80, 745–763 (2016).
Makarova, K. S. et al. Evolution and classification of the CRISPR–Cas systems. Nat. Rev. Microbiol. 9, 467–477 (2011).
Makarova, K. S. et al. An updated evolutionary classification of CRISPR–Cas systems. Nat. Rev. Microbiol. 13, 722–736 (2015).
Kazlauskiene, M., Kostiuk, G., Venclovas, Č., Tamulaitis, G. & Siksnys, V. A cyclic oligonucleotide signaling pathway in type III CRISPR-Cas systems. Science 357, 605–609 (2017).
Niewoehner, O. et al. Type III CRISPR–Cas systems produce cyclic oligoadenylate second messengers. Nature 548, 543–548 (2017).
Ofir, G. et al. DISARM is a widespread bacterial defence system with broad anti-phage activities. Nat. Microbiol. 3, 90–98 (2018).
Goldfarb, T. et al. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 34, 169–183 (2015).
Ofir, G. & Sorek, R. Contemporary phage biology: from classic models to new insights. Cell 172, 1260–1270 (2018).
Lopatina, A., Tal, N. & Sorek, R. Abortive infection: bacterial suicide as an antiviral immune strategy. Annu. Rev. Virol. 7, 1 (2020).
Chen, I. M. A. et al. IMG/M v.5.0: an integrated data management and comparative analysis system for microbial genomes and microbiomes. Nucleic Acids Res. 47, D666–D677 (2019).
Steinegger, M. & Söding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35, 1026–1028 (2017).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Steinegger, M. et al. HH-suite3 for fast remote homology detection and deep protein annotation. BMC Bioinform. 20, 473 (2019).
Berman, H. M. et al. The Protein Data Bank. Nucleic Acids Res. 28, 235–242 (2000).
El-Gebali, S. et al. The Pfam protein families database in 2019. Nucleic Acids Res. 47, D427–D432 (2019).
Price, M. N., Dehal, P. S. & Arkin, A. P. Fasttree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 26, 1641–1650 (2009).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44, W242–W245 (2016).
Acknowledgements
We thank the members of the Sorek laboratory for comments on earlier versions of this manuscript. A.M. was supported by a fellowship from the Ariane de Rothschild Women Doctoral Program and, in part, by the Israeli Council for Higher Education via the Weizmann Data Science Research Center. R.S. was supported in part by the Israel Science Foundation (personal grant no. 1360/16), the European Research Council (grant no. ERC-CoG 681203), the German Research Council (DFG) priority program SPP 2002 (grant no. SO 1611/1-1), the Israeli Council for Higher Education through the Weizmann Data Science Research Center, the Ernest and Bonnie Beutler Research Program of Excellence in Genomic Medicine, the Minerva Foundation with funding from the Federal German Ministry for Education and Research and the Knell Family Center for Microbiology.
Author information
Authors and Affiliations
Contributions
A.M. collected and analysed the data and wrote the paper. S.M. and G.A. were involved in the classification of CBASS systems. R.S. supervised the study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
R.S. is a scientific cofounder and consultant of BiomX, Pantheon Bioscience and Ecophage.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
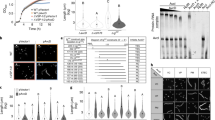
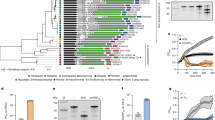
Extended Data Fig. 1 Phylogenetic analysis of oligonucleotide cyclases (CD-NTase) and their CBASS types.
The phylogenetic tree of all cyclases, as depicted and colored in refs (5 and 8) is presented in the center. Each clade is then expanded and presented in the periphery as a circular tree to increase resolution. Outer ring depicts the effector type; middle ring depicts the system type. Numbers next to each clade represent the bootstrap value for that node in the central tree.
Supplementary information
Supplementary Tables
Supplementary Tables 1–7.
Rights and permissions
About this article
Cite this article
Millman, A., Melamed, S., Amitai, G. et al. Diversity and classification of cyclic-oligonucleotide-based anti-phage signalling systems. Nat Microbiol 5, 1608–1615 (2020). https://doi.org/10.1038/s41564-020-0777-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-0777-y
This article is cited by
-
Reversible conjugation of a CBASS nucleotide cyclase regulates bacterial immune response to phage infection
Nature Microbiology (2024)
-
Phage defence system CBASS is regulated by a prokaryotic E2 enzyme that imitates the ubiquitin pathway
Nature Microbiology (2024)
-
Inhibitors of bacterial immune systems: discovery, mechanisms and applications
Nature Reviews Genetics (2024)
-
Conservation and similarity of bacterial and eukaryotic innate immunity
Nature Reviews Microbiology (2024)
-
Phages overcome bacterial immunity via diverse anti-defence proteins
Nature (2024)